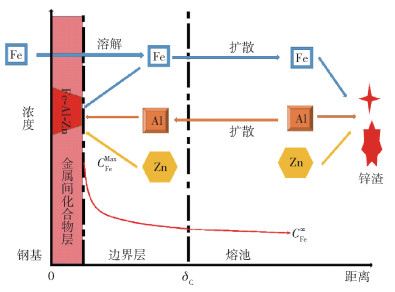
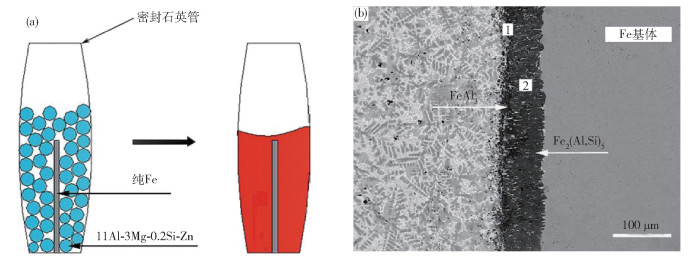
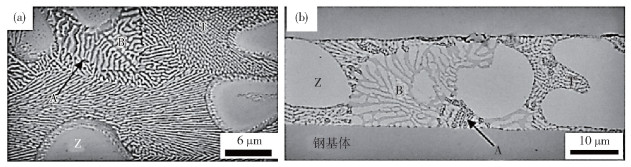
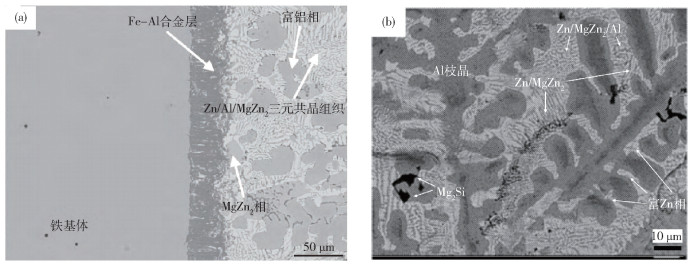
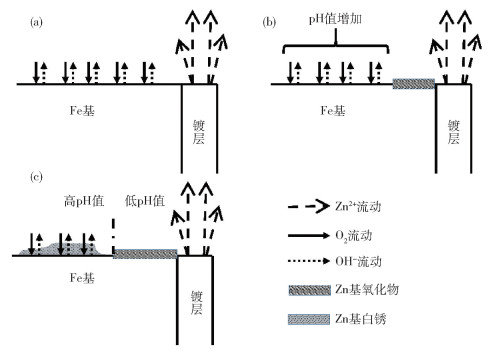
-
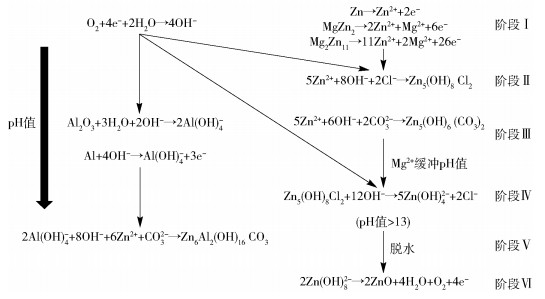
摘要: 從鋅鋁鎂鍍層的熔池界面反應、鍍層組織、表面和切邊腐蝕機理、腐蝕產物類型變化等方面, 對高耐蝕鋅鋁鎂鍍層的研究進展進行了詳細分析. 根據Al成分含量的不同, 將商用及實驗室鋅鋁鎂鍍層分為"低鋁"、"中鋁"和"高鋁"鋅鋁鎂三種類型: 不同類型的鋅鋁鎂鍍層的金屬間化合物層生長動力學存在差異, 為了控制鍍層厚度, 應合理控制浸鍍時間、溫度與熔池成分; 凝固組織也存在差異, "低鋁"與"中鋁"會析出Al或Zn初晶、Zn/MgZn2二元共晶組織、Zn/MgZn2/Al三元共晶組織, "高鋁"會產生富Al枝晶、枝晶間富Zn相、Mg2Si相、MgZn2相, 不產生共晶組織; 發生表面腐蝕時, "低鋁"與"中鋁"中MgZn2相先電離, 并生成堿性鋅鹽、雙層氫氧化物等致密的腐蝕產物, 抑制腐蝕; 發生切邊腐蝕時, 鋅鋁鎂會出現自修復現象, 在切邊鋼基或鍍層破損處形成堿性鋅鹽, 保護基體.Abstract: Zn-Al-Mg alloy coating, the most promising protective steel coating of the 21st century, is widely employed in construction, automotive, and other fields, due to its high surface and edge corrosion. In recent years, with the increasing demand for Zn-Al-Mg coating, a series of basic studies on Zn-Al-Mg coating materials has been carried out by foreign scholars, making significant progress and achievements. Simultaneously, the gap in the galvanizing industry between domestic and international has been expanding year by year. In order to gradually reduce gradually this gap with foreign countries, it is necessary to summarize and review the research achievements of foreign researchers. In this paper, the research progress into high corrosion resistant Zn-Al-Mg hot dip coatings was reviewed from the perspective of interfacial reactions in pots, coating structures, corrosion mechanisms of surface and cut edges, as well as corrosion product types of Zn-Al-Mg coatings. According to the range of Al content, laboratory and commercial Zn-Al-Mg coatings are divided into three types: "low-aluminum, " "medium-aluminum, " and "high-aluminum" coatings. There are differences in these coatings, including growth kinetics in the intermetallic compound layers of the different types of coating. In order to control the thickness of the coating, reasonable immersion time and temperature should be controlled. There are also differences in the solidification structures of the three types. Primary Al or Zn crystal, Zn/MgZn2 binary eutectics, and Zn/MgZn2/Al ternary eutectics would form in "low-aluminum" and "medium-aluminum, " while Al-rich dendrites, an intergranular Zn-rich phase, a Mg2Si phase, and a MgZn2 phase would occur with "high-aluminum" coatings. During surface corrosion in "low-aluminum" or "medium-aluminum, " the MgZn2 phase is ionized first, giving rise to a dense corrosion product to inhibit corrosion, such as basic zinc salt (BZS) or layered double hydroxide (LDH). Meanwhile, in the cut edge, a self-healing phenomenon occurs; the proposed explanation in this paper for this is Mg-containing corrosion product flowing or pH changing. However, there are some disputed aspects that need further study. In the hot dipping process, the intermetallic compound thickness should be controlled by the interfacial reaction at the steel/liquid melts through changing the molten bath temperature and holding time. The influence of Mg2Zn11 phase and MgZn2 on the corrosion resistance of Zn-Al-Mg coating is also controversial, so that the microstructure of Zn-Al-Mg coating needs further investigation for corrosion. Furthermore, a kinetic model of the corrosion process should be established to discover the controlling factors in the corrosion reaction, so that the life of the coating can be extended.
-
表 1 鋅鋁鎂金屬間化合物層生長動力學方程
Table 1. Growth kinetic equation of Zn-Al-Mg IMC layer
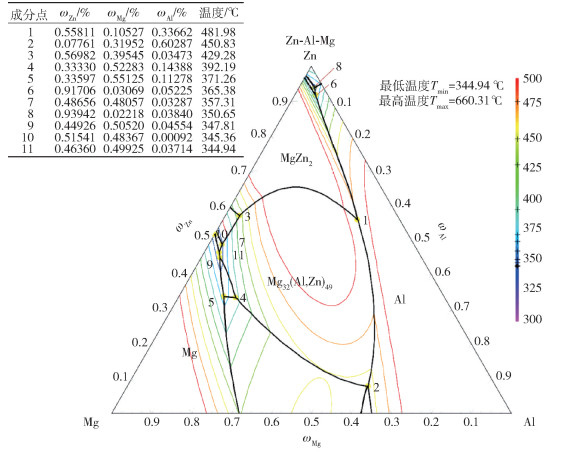
熔池 實驗溫度/℃ 金屬間化合物生長動力學方程 文獻來源 11Al-3Mg-Zn 510 Δx=3.1766·t0.6715 [4] 11Al-3Mg-0.2Si-Zn 510 Δx=0.1221·t0.5384 [4] 11Al-3Mg-0.2Si-Zn 480~650 $ \Delta x = 0.25 \cdot {{\rm{e}}^{ - \frac{{101}}{{RT}}}} \cdot {t^{0.5}} $ [5] 6Al-xMg-Zn (x=0, 1, 2, 3, 4, 5) 420~540 — [24] 6Al-3Mg-Zn — Δx=1.2716·t0.6035 [23] 11Al-1.5Mg-Zn 510 Δx=1.8699·t0.8109 [25] 11Al-4.5Mg-Zn 510 Δx=3.0554·t0.6709 [25] 表 2 鋅鋁鎂鍍層組織及代表產品牌號
Table 2. Coating structures and representative products
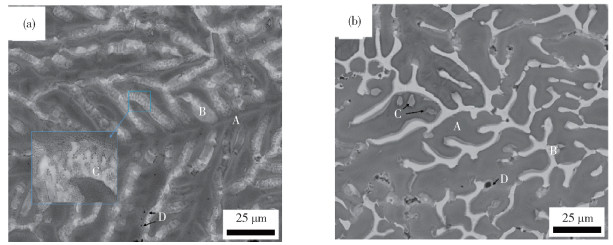
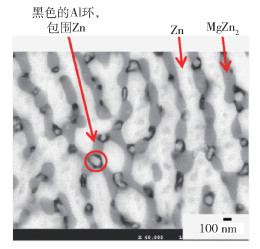
鋅鋁鎂類型 ωAl/% ωMg/% 鍍層組織類型[16] “低鋁” 1~5 1~2 初生Zn、Zn/MgZn2共晶組織、Zn/MgZn2/Al共晶組織 “中鋁” 6~13 3 初生Al、Zn/MgZn2共晶組織、Zn/MgZn2/Al共晶組織 “高鋁” 47~57 2 富Al枝晶、枝晶間富Zn相、Mg2Si相、MgZn2相 名稱 符號 化學式 雙層氫氧化物 LDH M(Ⅱ)xM(Ⅲ)y(A-)m(OH-)n·zH2O M(Ⅱ)=Zn2+, Mg2+; M(Ⅲ)=Al3+ A-=CO32-, Cl-, SO42- 羥基氯化鋅 ZHC Zn5(OH)8Cl2·H2O 羥基硫酸鋅 ZHS Zn4(OH)6SO4·nH2O,n=3~5 堿式碳酸鋅 HZ Zn5(OH)6(CO3)2·H2O 氧化鋅 ZnO ZnO 氫氧化鋅 Zn(OH)2 Zn(OH)2 氫氧化鎂 Mg(OH)2 Mg(OH)2 氫氧化鋁 Al(OH)3 Al(OH)3 259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Marder A R. The metallurgy of zinc-coated steel. Prog Mater Sci, 2000, 45(3): 191 doi: 10.1016/S0079-6425(98)00006-1 [2] Li F, Lü J S, Yang H G, et al. Research on ZnAlMg coated steel sheet. Steel Roll, 2013, 30(2): 45 doi: 10.3969/j.issn.1003-9996.2013.02.013李鋒, 呂家舜, 楊洪剛, 等. 鋅鋁鎂鍍層鋼板的研究進展. 軋鋼, 2013, 30(2): 45 doi: 10.3969/j.issn.1003-9996.2013.02.013 [3] Zhou Y J, Dai Y H, Jiang G R. Analysis of global patents technology on Zn-Al-Mg coated steel sheets in recent 20 years. Iron Steel, 2016, 51(11): 7 https://www.cnki.com.cn/Article/CJFDTOTAL-GANT201611002.htm周誼軍, 代云紅, 蔣光銳. 近20年全球鋅鋁鎂鍍層鋼板技術專利分析. 鋼鐵, 2016, 51(11): 7 https://www.cnki.com.cn/Article/CJFDTOTAL-GANT201611002.htm [4] Li K L, Liu Y, Tu H, et al. Effect of Si on the growth of Fe-Al intermetallic layer in Zn-11%Al-3%Mg coating. Surf Coat Technol, 2016, 306: 390 doi: 10.1016/j.surfcoat.2016.05.033 [5] Wang J H, Ma K, Peng H P, et al. Study on Fe-Al layers of Fe/(Zn-11% Al-3% Mg-0.2% Si) solid-liquid diffusion couples. Mater Manuf Processes, 2017, 32(11): 1290 doi: 10.1080/10426914.2017.1328117 [6] Yao C Z, Tay S L, Zhu T P, et al. Effects of Mg content on microstructure and electrochemical properties of Zn-Al-Mg alloys. J Alloys Compd, 2015, 645: 131 doi: 10.1016/j.jallcom.2015.05.010 [7] Wang Y B, Zeng J M. Effects of manganese addition on microstructures and corrosion behavior of hot-dip zinc coatings of hot-rolled steels. Surf Coat Technol, 2014, 245: 55 doi: 10.1016/j.surfcoat.2014.02.040 [8] Pistofidis N, Vourlias G, Konidaris S, et al. Microstructure of zinc hot-dip galvanized coatings used for corrosion protection. Mater Lett, 2006, 60(6): 786 doi: 10.1016/j.matlet.2005.10.013 [9] Duchoslav J, Arndt M, Steinberger R, et al. Nanoscopic view on the initial stages of corrosion of hot dip galvanized Zn-Mg-Al coatings. Corros Sci, 2014, 83: 327 doi: 10.1016/j.corsci.2014.02.027 [10] Schürz S, Luckeneder G H, Fleischanderl M, et al. Chemistry of corrosion products on Zn-Al-Mg alloy coated steel. Corros Sci, 2010, 52(10): 3271 doi: 10.1016/j.corsci.2010.05.044 [11] Volovitch P, Allely C, Ogle K. Understanding corrosion via corrosion product characterization: Ⅰ. Case study of the role of Mg alloying in Zn-Mg coating on steel. Corros Sci, 2009, 51(6): 1251 doi: 10.1016/j.corsci.2009.03.005 [12] Volovitch P, Vu T N, Allély C, et al. Understanding corrosion via corrosion product characterization: Ⅱ. Role of alloying elements in improving the corrosion resistance of Zn-Al-Mg coatings on steel. Corros Sci, 2011, 53(8): 2437 doi: 10.1016/j.corsci.2011.03.016 [13] Yuan X H, Lin Y, Zhang Q F. Cut-edge protection performance and corrosion resistance mechanisms of galvanized Zn-Al-Mg alloy coating. Chin J Nonferrous Met, 2015, 25(9): 2453 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201509018.htm袁訓華, 林源, 張啟富. 熱鍍鋅鋁鎂鍍層的切邊保護性能和耐腐蝕機理. 中國有色金屬學報, 2015, 25(9): 2453 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201509018.htm [14] Ueda K, Takahashi A, Kubo Y. Investigation of corrosion resistance of pre-painted Zn-11% Al-3% Mg-0.2% Si alloy coated steel sheet through outdoor exposure test in Okinawa. La Metall Ital, 2012(2): 13 http://www.researchgate.net/publication/279944140_Investigation_of_corrosion_resistance_of_pre-painted_Zn-11Al-3Mg-0.2Si_alloy_coated_steel_sheet_through_outdoor_exposure_test_in_Okinawa [15] Thébault F, Vuillemin B, Oltra R, et al. Investigation of self-healing mechanism on galvanized steels cut edges by coupling SVET and numerical modeling. Electrochim Acta, 2008, 53(16): 5226 doi: 10.1016/j.electacta.2008.02.066 [16] Xie Y X, Jin X Y, Wang L. Development and application of hot-dip galvanized zinc-aluminum-magnesium coating. J Iron Steel Res, 2017, 29(3): 167 https://www.cnki.com.cn/Article/CJFDTOTAL-IRON201703001.htm謝英秀, 金鑫焱, 王利. 熱浸鍍鋅鋁鎂鍍層開發及應用進展. 鋼鐵研究學報, 2017, 29(3): 167 https://www.cnki.com.cn/Article/CJFDTOTAL-IRON201703001.htm [17] Li S W. Study on Hot-Dip Technology and Corrosion Mechanism of Zn-Al-Mg-Si-RE Alloy[Dissertation]. Shenyang: Northeastern University, 2010李世偉. Zn-Al-Mg-Si-RE合金熱浸鍍工藝及其腐蝕機理的研究[學位論文]. 沈陽: 東北大學, 2010 [18] Li S W. Study on RE, Mg and Si in the modification of ZAM and Galvalume Coatings[Dissertation]. Shenyang: Northeastern University, 2013李世偉. RE、Mg、Si對ZAM和Galvalume鍍層的改性研究[學位論文]. 沈陽: 東北大學, 2013 [19] Hashimoto S, Nakazawa M. Zn-Al-Mg Coated Steel Sheet and Method of Producing Zn-Al-Mg Coated Steel Sheet: US Patent, 15/554, 451. 2018-8-23 [20] Su X P, Zhou J, Wang J H, et al. Thermodynamic analysis and experimental study on the oxidation of the Zn-Al-Mg coating baths. Appl Surf Sci, 2017, 396: 154 doi: 10.1016/j.apsusc.2016.11.043 [21] Zhang Y. Study on Smelting and Corrosion Mechanism of Zn-Al-Mg Alloys[Dissertation]. Beijing: China University of Mining and Technology, 2017張永. Zn-Al-Mg合金的熔煉及其腐蝕機理研究[學位論文]. 北京: 中國礦業大學, 2017 [22] Giorgi M L, Guillot J B, Nicolle R. Theoretical model of the interfacial reactions between solid iron and liquid zinc-aluminium alloy. J Mater Sci, 2005, 40(9-10): 2263 doi: 10.1007/s10853-005-1944-5 [23] Li K L. Study on the Effect of Alloy Elements on the Zn-11%Al Coating and Solidification Microstructure[Dissertation]. Changzhou: Changzhou University, 2016李凱良. 合金元素對Zn-11%Al鍍層及凝固組織影響的研究[學位論文]. 常州: 常州大學, 2016 [24] Liang L, Liu Y L, Liu Y, et al. Effect of Mg and temperature on Fe-Al alloy layer in Fe/(Zn-6% Al-x% Mg) solid-liquid diffusion couples. Surf Rev Lett, 2017, 24(Suppl 1): 1850010 doi: 10.1142/S0218625X18500105 [25] Li K L, Wu C J, Peng H P, et al. Effect of Mg on the solidification structure and growth of the intermetallic layer of a Zn-11%Al alloy coating. Chin J Eng, 2016, 38(8): 1123 https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201608011.htm李凱良, 吳長軍, 彭浩平, 等. Mg對Zn-11%Al合金鍍層凝固組織及合金層生長的影響. 工程科學學報, 2016, 38(8): 1123 https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201608011.htm [26] Li S W, Gao B, Tu G F, et al. Effects of magnesium on the microstructure and corrosion resistance of Zn-55Al-1.6Si coating. Constr Build Mater, 2014, 71: 124 doi: 10.1016/j.conbuildmat.2014.08.023 [27] Azevedo M S, Allély C, Ogle K, et al. Corrosion mechanisms of Zn (Mg, Al) coated steel: 2. the effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros Sci, 2015, 90: 482 doi: 10.1016/j.corsci.2014.07.042 [28] Honda K, Yamada W, Ushioda K. Solidification structure of the coating layer on hot-dip Zn-11% Al-3% Mg-0.2% Si-coated steel sheet. Mater Trans, 2008, 49(6): 1395 doi: 10.2320/matertrans.MRA2008009 [29] Yamada W, Tanaka K, Ushioda K, et al. Solidification structure of coating layer in hot-dip Zn-11% Al-3% Mg-0.2% Si-coated steel sheet and phase diagram of the system. Nippon Steel Tech Rep, 2013, 102: 37 http://ci.nii.ac.jp/naid/40019264419 [30] De Bruycker E, Zermout Z, De Cooman B C. Zn-Al-Mg coatings: thermodynamic analysis and microstructure related properties. Mater Sci Forum, 2007, 539-5423: 1276 [31] Azevedo M S, Allély C, Ogle K, et al. Corrosion mechanisms of Zn (Mg, Al) coated steel in accelerated tests and natural exposure: 1. the role of electrolyte composition in the nature of corrosion products and relative corrosion rate. Corros Sci, 2015, 90: 472 [32] Lü J S, Li F, Yang H G, et al. Research on coating microstructure and corrosion behavior of galvanized Zn-Al-Mg coated steel sheet. J Mater Eng, 2012(10): 89 doi: 10.3969/j.issn.1001-4381.2012.10.019呂家舜, 李鋒, 楊洪剛, 等. 熱浸鍍鋅鋁鎂鋼板鍍層組織及腐蝕性能研究. 材料工程, 2012(10): 89 doi: 10.3969/j.issn.1001-4381.2012.10.019 [33] Oh M S, Kim S H, Kim J S, et al. Surface and cut-edge corrosion behavior of Zn-Mg-Al alloy-coated steel sheets as a function of the alloy coating microstructure. Met Mater Int, 2016, 22(1): 26 doi: 10.1007/s12540-015-5411-9 [34] Krieg R, Rohwerder M, Evers S, et al. Cathodic self-healing at cut-edges: the effect of Zn2+ and Mg2+ ions. Corros Sci, 2012, 65: 119 doi: 10.1016/j.corsci.2012.08.008 -




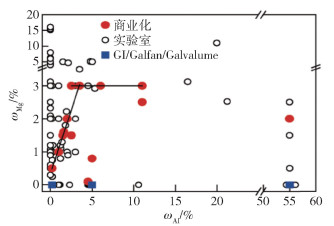
 下載:
下載: