-
摘要: 非氧化物陶瓷作為一種性能優異的高溫結構材料,廣泛應用于冶金、化工等高溫行業。在實際應用過程中氧化和應力耦合的服役環境加速了非氧化物陶瓷高溫性能失效最終降低服役壽命,甚至引發安全事故。因此研究非氧化物陶瓷在復雜服役環境下尤其應力條件下的氧化進程尤為重要,建立此條件下的氧化動力學模型是掌握材料實際服役行為規律的有效手段。本文對比了非氧化物陶瓷分別在無應力和應力條件下的氧化機理和相應動力學模型,通過對不同模型的應用對比分析,從量化角度明確了應力對非氧化物陶瓷氧化過程的影響,在此基礎上初步建立了考慮應力的非氧化物陶瓷氧化動力學模型,為進一步揭示非氧化物陶瓷在復雜條件下的服役行為提供科學模型,為提高材料服役壽命提供有效理論指導。Abstract: Nonoxide ceramics (NOCs) as representative high-temperature structural materials are widely applied in various key fields, such as metallurgy, electric power, and chemical industry, owing to combined excellent characteristics including high strength, lightweight, good thermal shock resistance, and erosion resistance. In practical applications, NOCs are often exposed to high temperatures containing oxygen; thus, they are inevitably confronted with the oxidation issue. In addition, NOCs are mostly used as lining materials for high-temperature containers and transmission components for high-temperature devices, in which they are also subjected to external loads. Simultaneously, internal stress will be generated inside the oxide film during oxidation, owing to the density difference and thermal expansion coefficient difference between the oxidation products and substrate. The coupled effect of oxidation and complex stresses accelerates the degradation of high-temperature performance and ultimately reduces the service life of NOCs, even causing severe industrial accidents. Therefore, studying the oxidation of NOCs under complex conditions is essential. However, limited by the high temperature and long serving time, an experimental approach to the oxidation of NOCs remains a challenge. By comparison, kinetic models based on specific reaction principles and different assumptions have become an effective tool for understanding and analyzing the oxidation of NOCs. This article compares the oxidation mechanism and corresponding kinetic models of NOCs under stressfree and stress conditions. Through comparing and analyzing the application effects of different models, the effect of stress on oxidation of NOCs is determined from a quantitative point, and the oxidation kinetic models of NOCs considering stress are preliminarily established, which can provide a scientific model for further recognition of service behavior of NOCs under complex conditions and provide effective theoretical guidance for improvement of the service life of materials.
-
Key words:
- nonoxide ceramics /
- oxidation /
- diffusion /
- stress /
- kinetic model
-
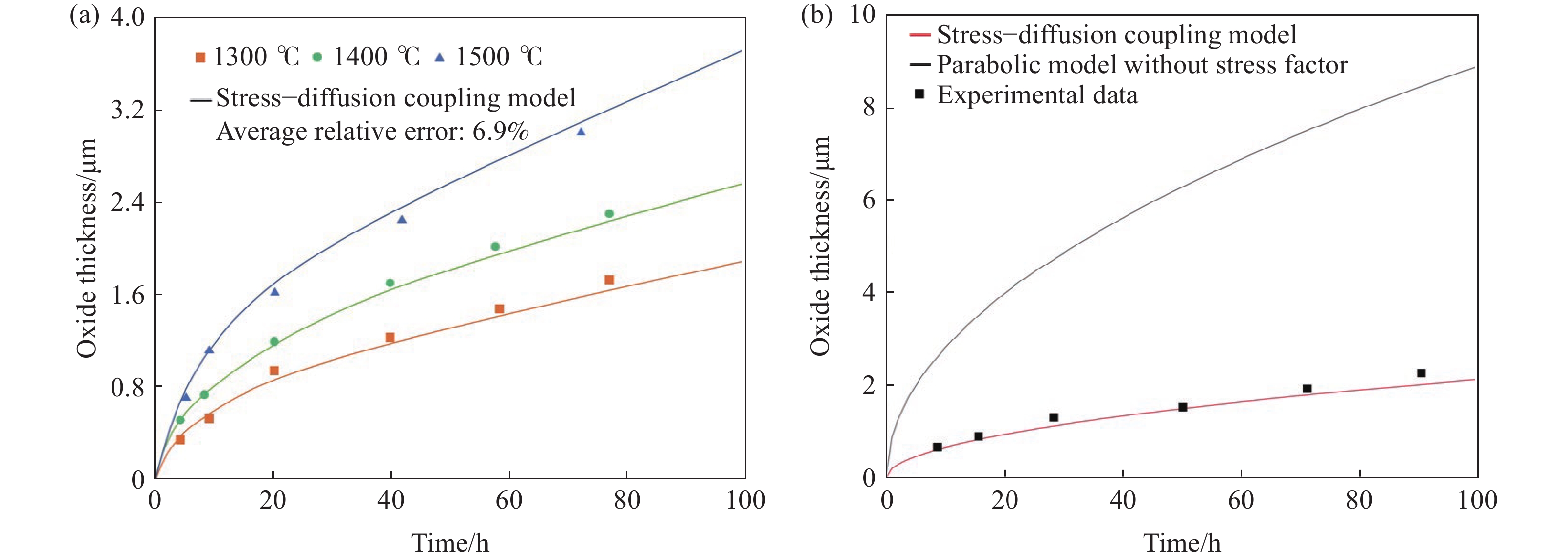
圖 4 應力?擴散耦合模型的應用。(a)計算結果和不同溫度下片狀SiC氧化的實驗數據對比圖[33];(b)應力?擴散耦合模型與未考慮內應力的拋物線模型計算結果和1300 ℃下片狀SiC氧化的實驗數據對比圖[33]
Figure 4. Application of the stress-diffusion coupling model: (a) comparison of calculation curves using the stress–diffusion coupling model and experimental data of bulk SiC oxidized at different temperatures[33]; (b) comparison of calculation curves using the stress–diffusion coupling model and parabolic model without the stress factor and experimental data of bulk SiC oxidized at 1300 ℃[33]
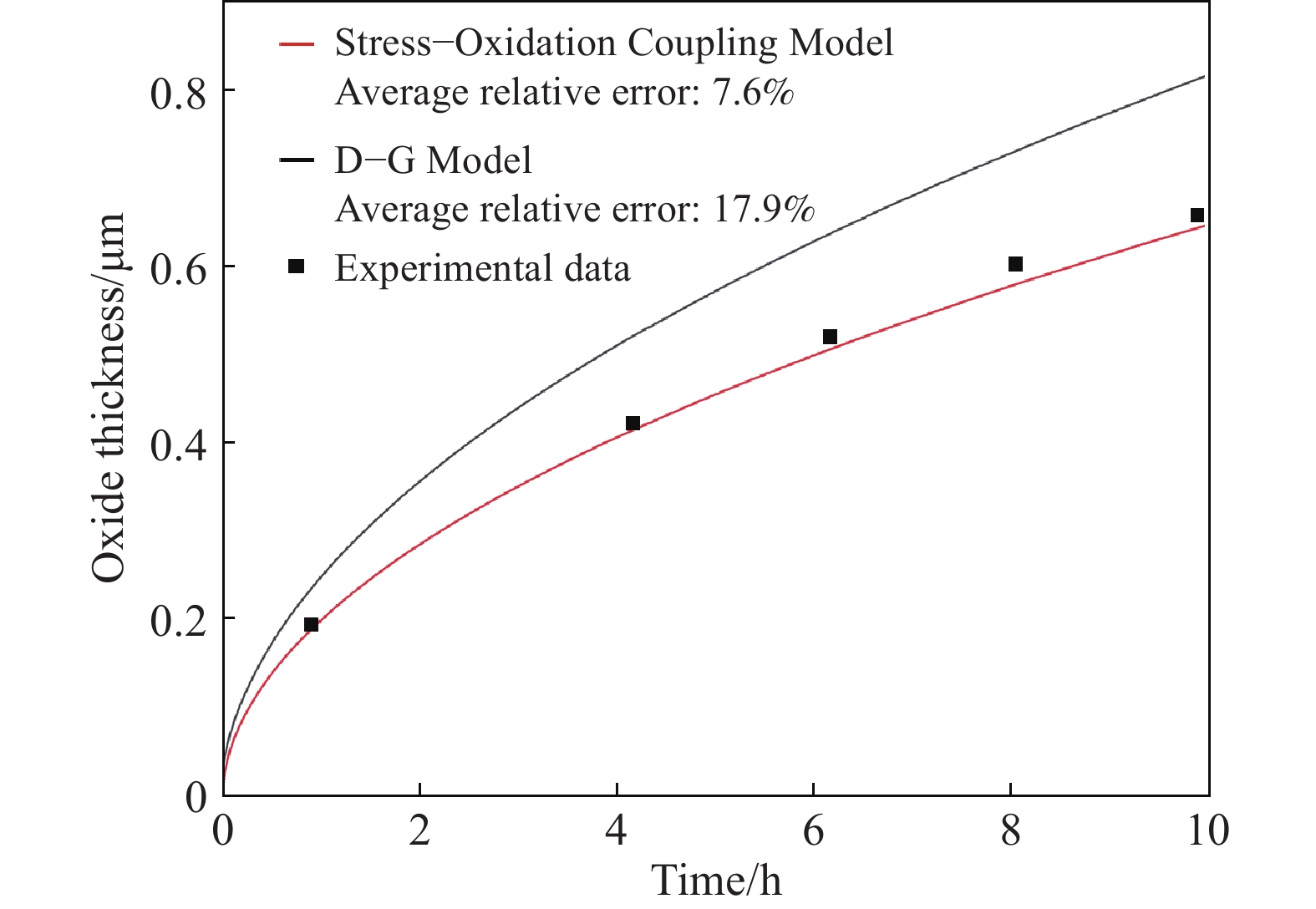
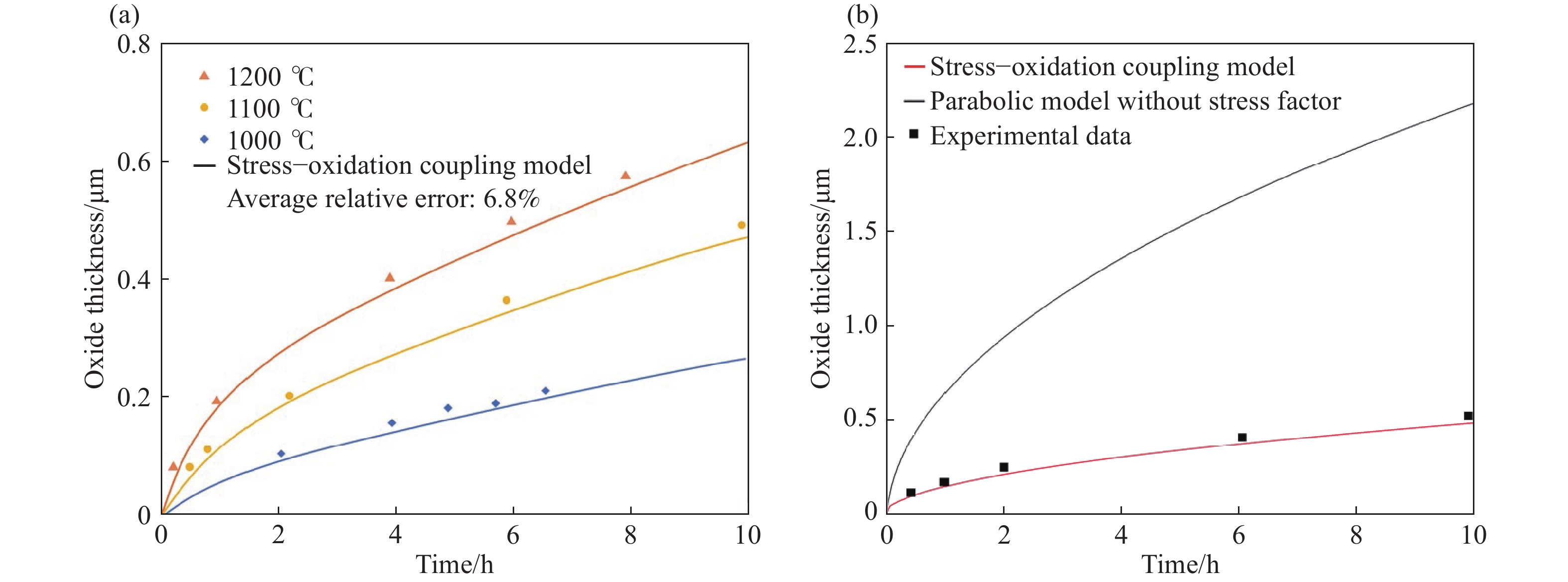
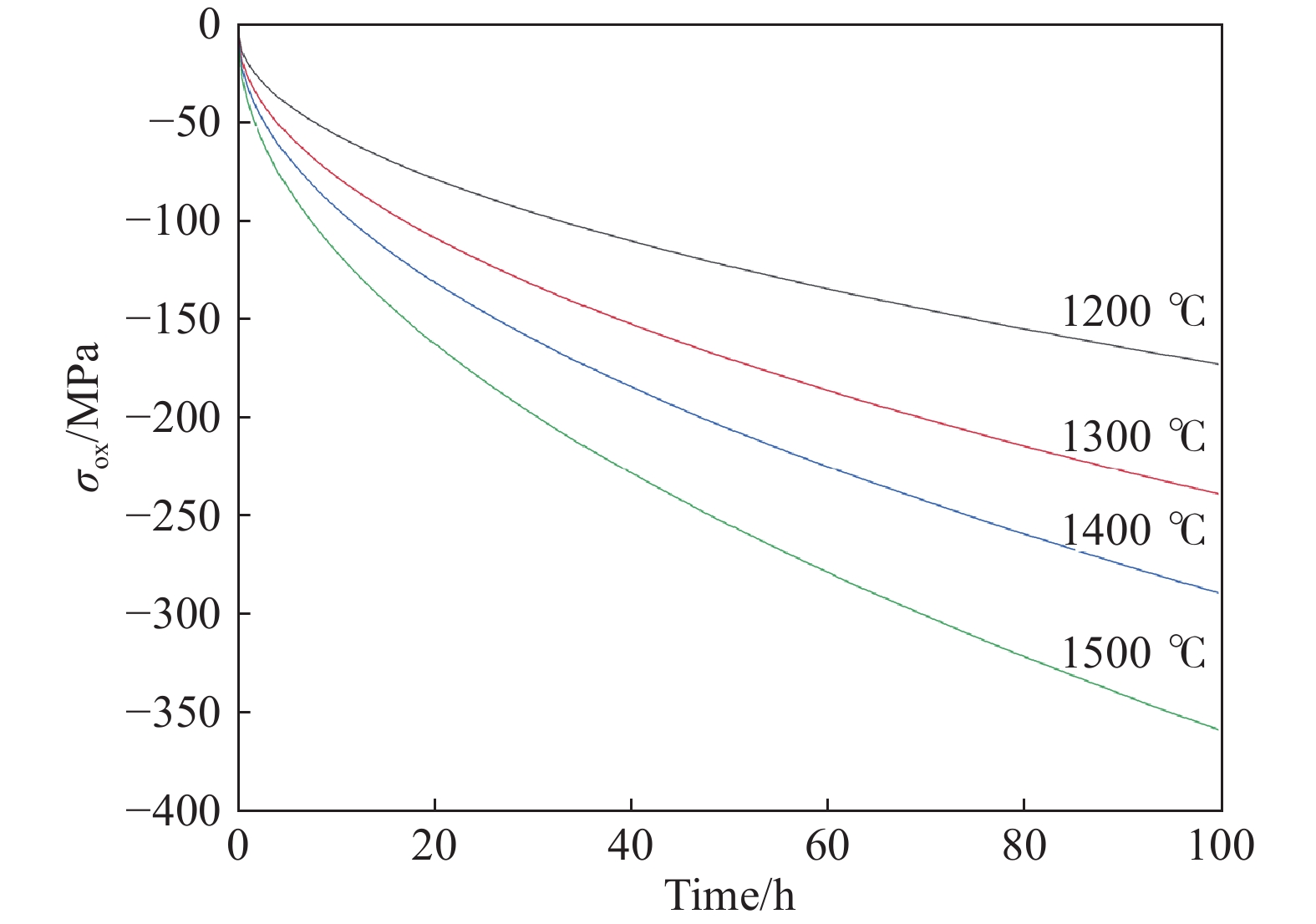
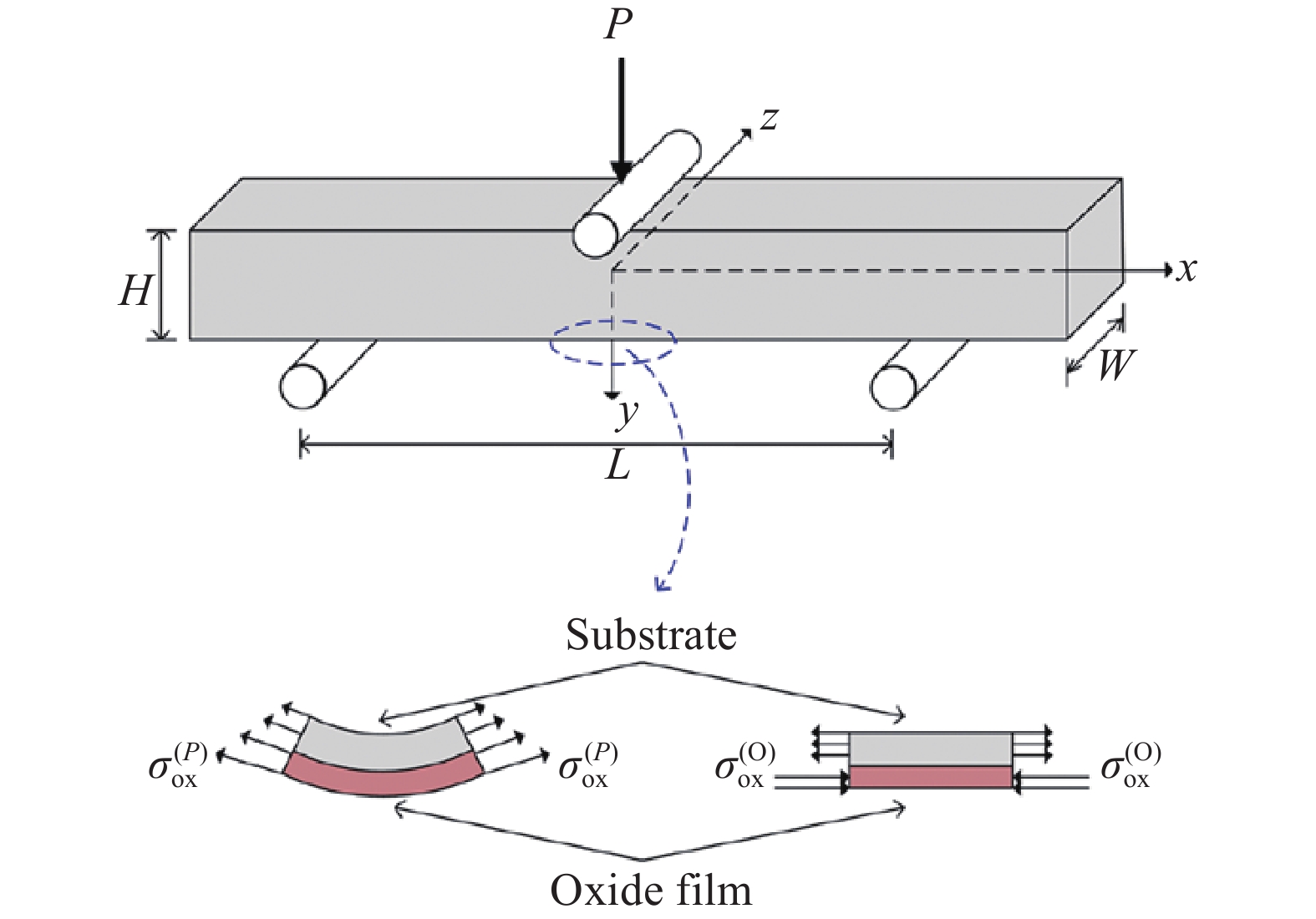
圖 6 應力?氧化耦合模型的應用。(a)模型計算結果和不同溫度下Si片氧化的實驗數據對比圖[7];(b)模型中考慮/不考慮應力因素的計算結果和1100 ℃下Si片氧化實驗數據對比圖[7]
Figure 6. Application of the stress-oxidation coupling model: (a) comparison of calculation curves using the stress–oxidation coupling model and the experimental data of bulk Si oxidized at different temperatures[7]; (b) comparison of calculation curves using the stress–oxidation coupling model and parabolic model without the stress factor and the experimental data of bulk Si oxidized at 1100 ℃[7]
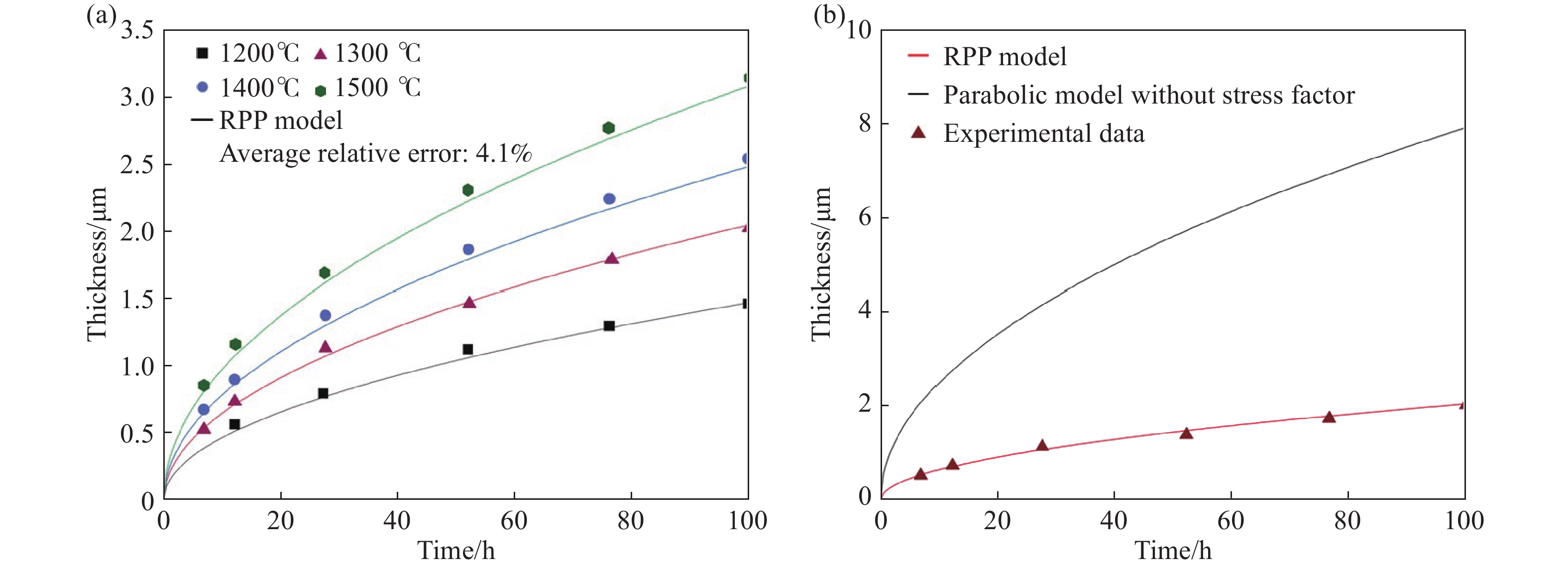
圖 8 RPP模型的應用。(a)模型計算結果和不同溫度下片狀SiC氧化實驗數據的對比圖[33];(b)RPP模型與未考慮內應力的拋物線模型計算結果和1300 ℃下片狀SiC氧化實驗數據的對比圖[33]
Figure 8. Application of RPP model: (a) comparison of calculation and prediction curves using the RPP model and experimental data of bulk SiC oxidized at different temperatures[33]; (b) comparison of calculation curves using the RPP model and parabolic model without the stress factor and experimental data of bulk SiC oxidized at 1300 ℃[33]
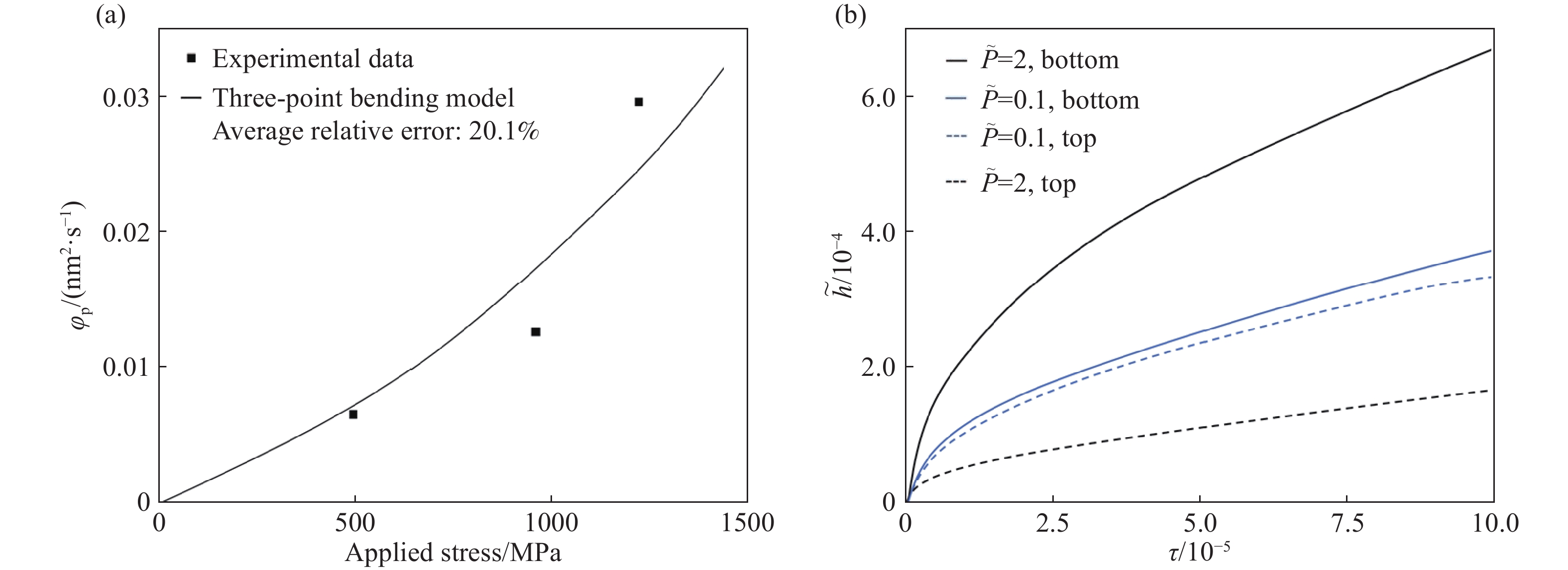
圖 10 三點彎曲模型的應用(
$ \widetilde h $ ,$ \tau $ 分別表示量綱為一的氧化膜厚度和量綱為一的時間)。(a)模型計算結果和800 ℃下片狀SiC氧化的實驗數據對比圖[37];(b)施加不同載荷對試樣頂部和底部氧化影響對比圖Figure 10. Application of the three-point bending model (
$ \widetilde h $ ,$ \tau $ represent the dimensionless oxide film thickness and dimensionless time, respectively): (a) comparison of calculation curves using the three-point bending model and experimental data of bulk SiC oxidized at 800 ℃[37]; (b) comparison of the influence of different applied loads on the oxidation of the top and bottom of the sample259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Wang E H, Chen J H, Hou X M. Current research and latest developments on refractories used as ladle linings. Chin J Eng, 2019, 41(6): 695王恩會, 陳俊紅, 侯新梅. 鋼包工作襯用耐火材料的研究現狀及最新進展. 工程科學學報, 2019, 41(6):695 [2] Zhen Q, Lu F, Song S L, et al. Influence of nano-SiC on the graphitization and oxidation resistance of C/C composites. Chin J Eng, 2017, 39(1): 81甄強, 魯飛, 宋紹雷, 等. 納米SiC對C/C復合材料石墨化與抗氧化性能的影響規律. 工程科學學報, 2017, 39(1):81 [3] Zhao C Y, Wang E H, Hou X M. Research progress on the oxidation mechanism and kinetics of a SiC semiconductor with different crystal surfaces. Chin J Eng, 2021, 43(5): 594趙春陽, 王恩會, 侯新梅. SiC半導體不同晶面氧化機理及動力學的研究進展. 工程科學學報, 2021, 43(5):594 [4] Jander W. Reactions in solid states at high temperature. I. Announcement the rate of reaction in endothermic conversions. Z Anorg Allgem Chem, 1927, 163(1): 1 (Jander W. Reaktionen im festen zustande bei höheren temperaturen. I. Reaktionsgeschwindigkeiten endotherm verlaufender umsetzungen. Z Anorg Allgem Chem, 1927, 163(1): 1) [5] Khawam A, Flanagan D R. Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B, 2006, 110(35): 17315 doi: 10.1021/jp062746a [6] Carter R E. Kinetic model for solid-state reactions. J Chem Phys, 1961, 34(6): 2010 doi: 10.1063/1.1731812 [7] Deal B E, Grove A S. General relationship for the thermal oxidation of silicon. J Appl Phys, 1965, 36(12): 3770 doi: 10.1063/1.1713945 [8] Tedmon C S. The effect of oxide volatilization on the oxidation kinetics of Cr and Fe–Cr alloys. J Electrochem Soc, 1966, 113(8): 766 doi: 10.1149/1.2424115 [9] Opila E J, Jacobson N S. SiO(g) formation from SiC in mixed oxidizing-reducing gases. Oxid Met, 1995, 44(5-6): 527 doi: 10.1007/BF01051042 [10] Beke D L, Szabó I A, Erdélyi Z, et al. Diffusion-induced stresses and their relaxation. Mater Sci Eng:A, 2004, 387-389: 4 doi: 10.1016/j.msea.2004.01.065 [11] Bull S J. Modeling of residual stress in oxide scales. Oxid Met, 1998, 49: 1 doi: 10.1023/A:1018822222663 [12] Clarke D R. Stress generation during high-temperature oxidation of metallic alloys. Curr Opin Solid State Mater Sci, 2002, 6(3): 237 doi: 10.1016/S1359-0286(02)00074-8 [13] Li X Y, Ermakov A, Amarasinghe V, et al. Oxidation induced stress in SiO2/SiC structures. Appl Phys Lett, 2017, 110(14): 141604 doi: 10.1063/1.4979544 [14] Chen X Y, Wen Y B, Chen H, et al. Stress–oxidation research progress of easily oxidized materials. Refractories, 2018, 52(1): 75 doi: 10.3969/j.issn.1001-1935.2018.01.019陳曉雨, 文鈺斌, 陳浩, 等. 易氧化材料的應力–氧化研究進展. 耐火材料, 2018, 52(1):75 doi: 10.3969/j.issn.1001-1935.2018.01.019 [15] Dong X L, Fang X F, Feng X, et al. Diffusion and stress coupling effect during oxidation at high temperature. J Am Ceram Soc, 2013, 96(1): 44 doi: 10.1111/jace.12105 [16] Yue M K, Dong X L, Fang X F, et al. Effect of interface reaction and diffusion on stress-oxidation coupling at high temperature. J Appl Phys, 2018, 123(15): 155301 doi: 10.1063/1.5025149 [17] Chou K C. A kinetic model for oxidation of Si–Al–O–N materials. J Am Ceram Soc, 2006, 89(5): 1568 doi: 10.1111/j.1551-2916.2006.00959.x [18] Chou K C, Hou X M. Kinetics of high-temperature oxidation of inorganic nonmetallic materials. J Am Ceram Soc, 2009, 92(3): 585 doi: 10.1111/j.1551-2916.2008.02903.x [19] Wang E H, Chen J H, Hu X J, et al. New perspectives on the gas–solid reaction of α-Si3N4 powder in wet air at high temperature. J Am Ceram Soc, 2016, 99(8): 2699 doi: 10.1111/jace.14274 [20] Wang X D. The Performance and Structure of AlON and MeAlON Ceramics [Dissertation]. Beijing: University of Science and Technology Beijing, 2001王習東. AlON及MeAlON陶瓷的性能與結構[學位論文]. 北京: 北京科技大學, 2001 [21] Chou K C, Xu K D. A new model for hydriding and dehydriding reactions in intermetallics. Intermetallics, 2007, 15(5-6): 767 doi: 10.1016/j.intermet.2006.10.004 [22] Hou X M, Chou K C, Li F S. Some new perspectives on oxidation kinetics of SiAlON materials. J Eur Ceram Soc, 2008, 28(6): 1243 doi: 10.1016/j.jeurceramsoc.2007.09.041 [23] Ramberg C E, Cruciani G, Spear K E, et al. Passive-oxidation kinetics of high-purity silicon carbide from 800 ℃ to 1100 ℃. J Am Ceram Soc, 1996, 79(11): 2897 doi: 10.1111/j.1151-2916.1996.tb08724.x [24] Opila E J. Variation of the oxidation rate of silicon carbide with water-vapor pressure. J Am Ceram Soc, 1999, 82(3): 625 [25] Wang Y G, Ma B S, Li L L, et al. Oxidation behavior of ZrB2?SiC?TaC ceramics. J Am Ceram Soc, 2012, 95(1): 374 doi: 10.1111/j.1551-2916.2011.04945.x [26] Nasiri N A, Patra N, Ni N, et al. Oxidation behaviour of SiC/SiC ceramic matrix composites in air. J Eur Ceram Soc, 2016, 36(14): 3293 doi: 10.1016/j.jeurceramsoc.2016.05.051 [27] Zhou C H. High Temperature Oxidation Behaviors of Several Metallic Materials in the Presence of External Compressive Stress or High Magnetic Field [Dissertation]. Dalian: Dalian University of Technology, 2009周長海. 幾種金屬材料在壓應力及強磁場下的高溫氧化[學位論文]. 大連: 大連理工大學, 2009 [28] Barvosa-Carter W, Aziz M J, Gray L J, et al. Kinetically driven growth instability in stressed solids. Phys Rev Lett, 1998, 81(7): 1445 doi: 10.1103/PhysRevLett.81.1445 [29] Haftbaradaran H, Gao H J, Curtin W A. A surface locking instability for atomic intercalation into a solid electrode. Appl Phys Lett, 2010, 96(9): 091909 doi: 10.1063/1.3330940 [30] Xiao X, Liu P, Verbrugge M W, et al. Improved cycling stability of silicon thin film electrodes through patterning for high energy density lithium batteries. J Power Sources, 2011, 196(3): 1409 doi: 10.1016/j.jpowsour.2010.08.058 [31] Coffin H, Bonafos C, Schamm S, et al. Oxidation of Si nanocrystals fabricated by ultralow-energy ion implantation in thin SiO2 layers. J Appl Phys, 2006, 99(4): 044302 doi: 10.1063/1.2171785 [32] Limarga A M, Wilkinson D S, Weatherly G C. Modeling of oxidation-induced growth stresses. Scr Mater, 2004, 50(12): 1475 doi: 10.1016/j.scriptamat.2004.03.001 [33] Ogbuji L U J T, Opila E J. A comparison of the oxidation kinetics of SiC and Si3N4. J Electrochem Soc, 1995, 142(3): 925 doi: 10.1149/1.2048559 [34] Suo Y H, Shen S P. General approach on chemistry and stress coupling effects during oxidation. J Appl Phys, 2013, 114(16): 164905 doi: 10.1063/1.4826530 [35] Haftbaradaran H, Song J, Curtin W A, et al. Continuum and atomistic models of strongly coupled diffusion, stress, and solute concentration. J Power Sources, 2011, 196(1): 361 doi: 10.1016/j.jpowsour.2010.06.080 [36] Dong X L, Fang X F, Feng X, et al. Oxidation at high temperature under three-point bending considering stress-diffusion coupling effects. Oxid Met, 2016, 86(1-2): 125 doi: 10.1007/s11085-016-9626-z [37] Gauthier W, Pailler F, Lamon J, et al. Oxidation of silicon carbide fibers during static fatigue in air at intermediate temperatures. J Am Ceram Soc, 2009, 92(9): 2067 doi: 10.1111/j.1551-2916.2009.03180.x -




 下載:
下載: