Research progress and development trends in heterogeneous Fenton-like catalysts for degradation of antibiotics in wastewater
-
摘要: 水中抗生素具有成分復雜、毒性高和難于生物降解等特點,成為近些年水處理領域的研究熱點。均相Fenton氧化技術(Fe2+/H2O2體系)因其反應快速、簡單高效而備受青睞。而異相類Fenton氧化技術采用鐵基固體催化劑代替液相Fe2+,能夠有效減少含鐵污泥的生成,同時拓寬反應的pH值范圍,且催化劑可以回收利用,在近些年得到了快速發展,將其應用于抗生素的降解也取得了理想的效果。從異相類Fenton催化原理出發,綜述了異相類Fenton催化劑降解抗生素的研究進展。基于異相類Fenton催化劑的核心問題,重點闡述了改善催化性能的方法、措施以及新的觀點。針對異相類Fenton技術降解抗生素存在的問題提出了今后的發展方向。
-
關鍵詞:
- 抗生素 /
- 水處理 /
- Fenton氧化 /
- 異相類Fenton催化劑 /
- 催化性能
Abstract: China is one of the major antibiotic producing and consuming countries in the world and demand for and output of antibiotics are increasing year by year. The wastewater produced during the production of antibiotics is of complex composition, high chemical oxygen demand, high concentrations of toxic and harmful substances and strong resistance to biodegradation. This is problematic for the process of pharmaceutical wastewater treatment. Additionally, due to the extensive use of antibiotics, a variety of antibiotics are constantly released into the environment, with adverse effects on aquatic organisms. Although antibiotic concentrations in wastewater are low, the accumulation of low-dose, long-duration antibiotics can lead to the development of drug-resistant strains that threaten human health and the entire ecosystem. Therefore, how to effectively remove antibiotic residues in water is an important challenge toward ensuring the safety of water quality, the environment, and ecology. Advanced oxidation technology (AOP), which has an extremely high oxidation potential, can generate hydroxyl radicals in the reaction process, and can degrade organic compounds rapidly without secondary pollution. Therefore, it exhibits clear advantages in the treatment of antibiotics in water. As a kind of AOP, homogeneous Fenton oxidation technology (Fe2+/H2O2 system) has attracted considerable attention owing to its rapid reaction, simplicity, and high degradation efficiency. Heterogeneous Fenton-like oxidation technology using an iron-based solid catalyst instead of Fe2+ ions can effectively reduce the formation of iron-containing sludge and broaden the pH reaction range to overcome the shortcomings of Homogeneous Fenton. Moreover, recycling of the catalyst has been developed rapidly in recent years and has achieved ideal results when applied to the degradation of antibiotics. In this paper, research progress in heterogeneous Fenton-like catalysts for degradation of antibiotics was reviewed. Based on the core issues of heterogeneous Fenton-like catalysts, the methods, measures, and new viewpoints for improving catalytic performance were expounded upon. Aiming at the problems of heterogeneous Fenton-like technology in degrading antibiotics, future development directions were presented. -
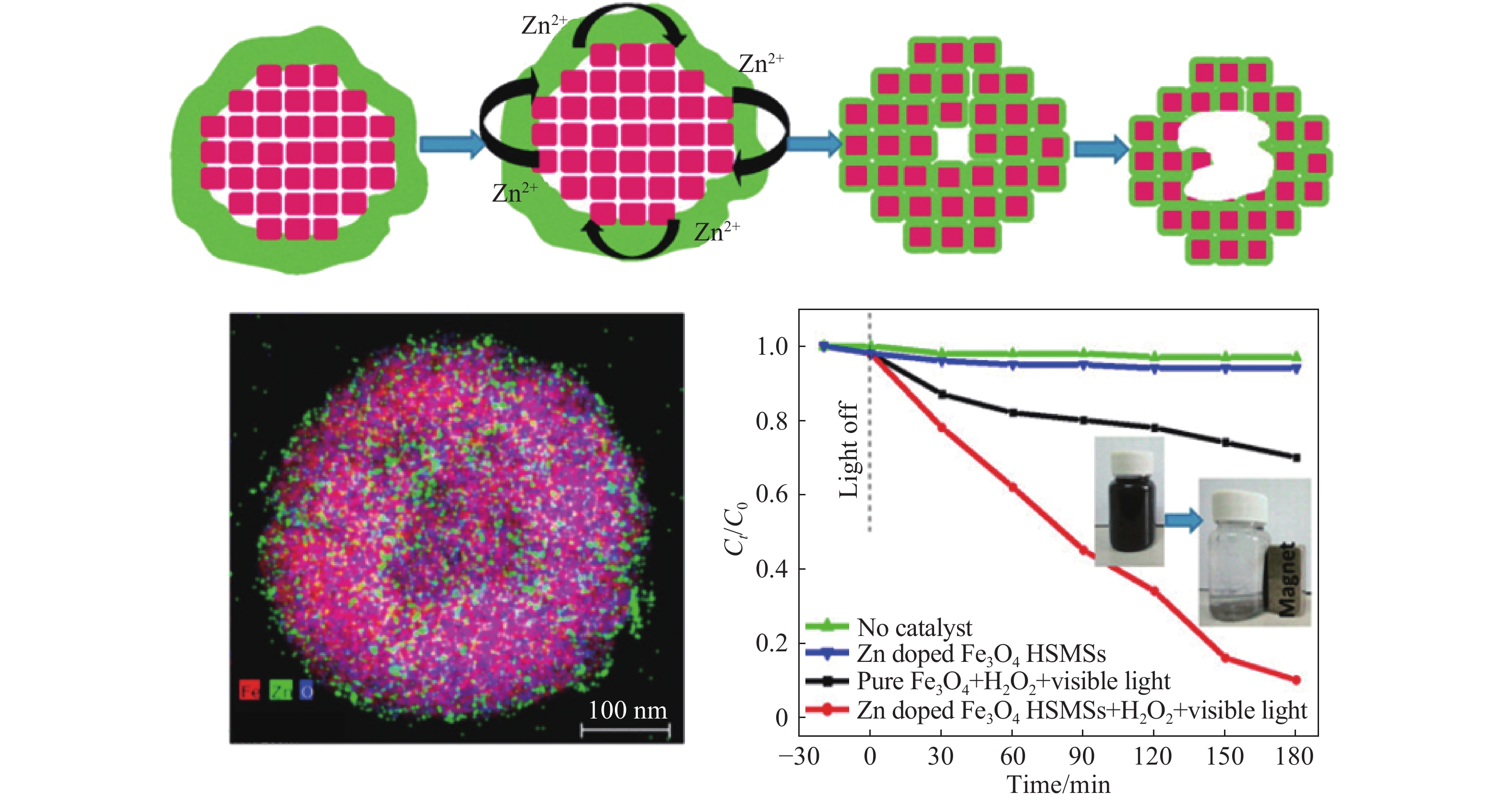
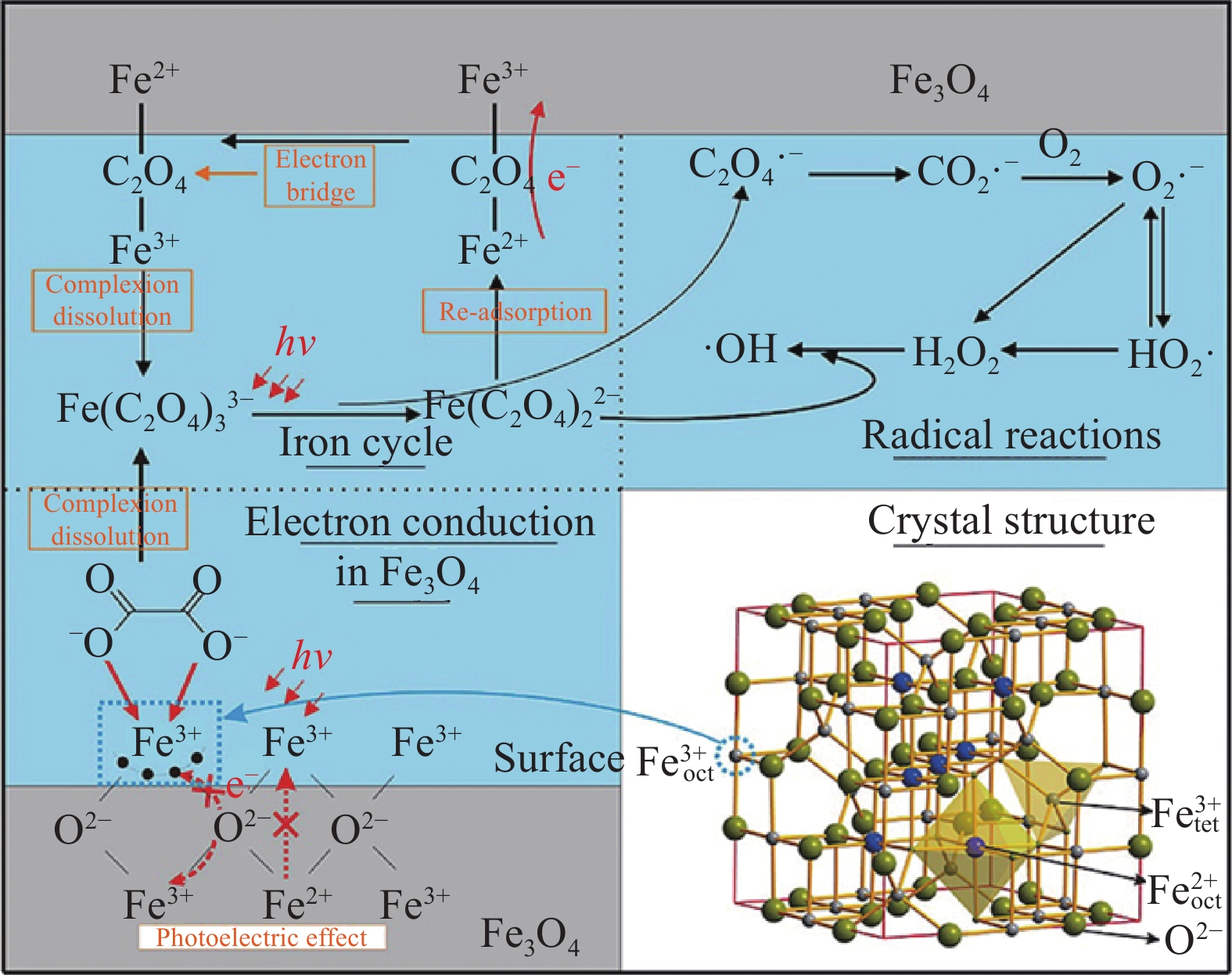
圖 2 Zn摻雜Fe3O4空心亞微球介晶形成過程示意圖及不同條件下對頭孢氨芐的降解(插圖為外加磁場下回收催化劑)[20]
Figure 2. Schematic of the formation process for Zn-doped Fe3O4 hollow submicrosphere mesocrystals and degradation of cephalexin under different conditions (illustration shows the recovery of the catalyst under an external magnetic field)[20]
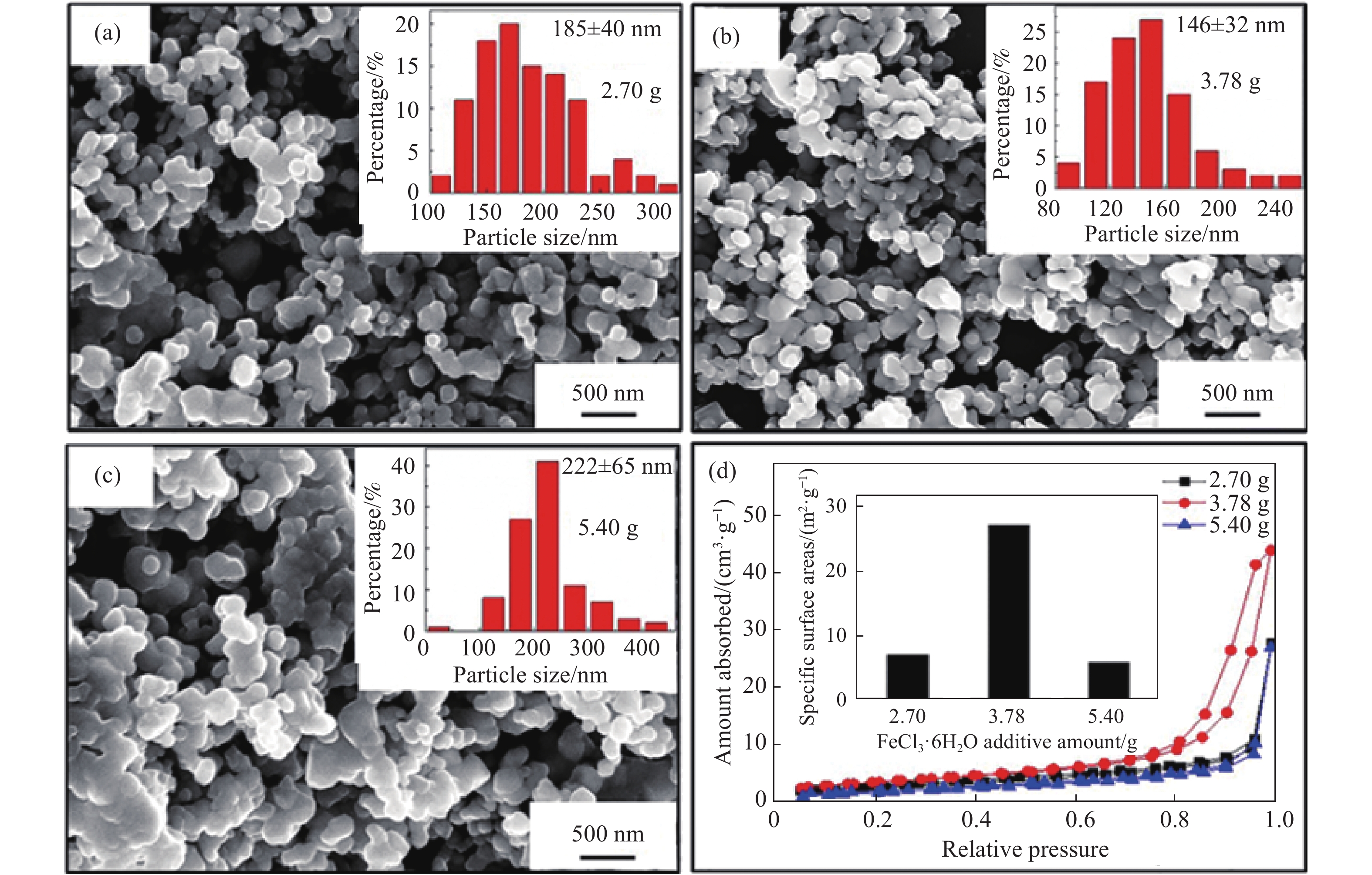
圖 5 (a~c)不同FeCl3·6H2O添加量制備產物的SEM圖(插圖為粒徑分布圖)和(d)不同產物的N2吸附?脫附等溫線和BET比表面積圖[70]
Figure 5. (a?c) SEM images of products prepared with different FeCl3·6H2O additions (insets are particle size distribution diagrams) and (d)N2 adsorption?desorption isotherms and BET-specific surface area diagrams of different products[70]
表 1 摻雜與復合型異相類Fenton催化劑降解水中抗生素研究實例
Table 1. Study of degradation of antibiotics in water with doped and composite heterogeneous Fenton-like catalysts
Catalyst Antibiotic Reaction condition Degradation effect Reusability Fe/Si codoped TiO2[18] Metronidazole 0.0006% 0.3 g catalyst, 10 mmol·L?1 H2O2, 220 W Xenon lamp,
pH 7.0, 25 ℃93%/50 min Fifth cycles /
80%WMoO-x[19] Tetracycline 400 μmol·L?1 0.8 g?L–1 catalyst, 20 mmol·L?1 H2O2, pH 4.0, 25 ℃ 91.75%/60 min Fifth cycles/
89.9%Zn-doped Fe3O4[20] Cephalexin 10 mg?L?1 0.10 g catalyst, 0.1 mL H2O2,350 W Xenon lamp 90%/180 min,72.3% TOC/180 min α-Fe2O3@g-C3N4[10] Tetracycline 40 mg?L?1 0.5 g?L?1 catalyst, 10 mmol·L?1 H2O2, 100 W LED lamp,
pH 5.5, 25 ℃92%/60 min Fifth cycles/
80%FeCu@C[48] Sulfamethazine 20 mg?L?1 0.25 g?L?1 catalyst, 1.5 mmol·L?1 H2O2, pH 3.0, 25 ℃ 100% /90 min,72.3% TOC/240 min Third cycle/
90.1%TiO2/Fe3O4[9] Tetracycline 50 mg?L?1 0.3 g?L?1 catalyst, 10 mmol·L?1 H2O2, 10 W UVC lamp,
23 ± 1 ℃, pH 7.098%/60 min,64.2% TOC/120 min Fifth cycles/
90%rGO-APTMS-FMBO[49] Sulfamethoxazole
0.039 mmol·L?10.2 g?L?1 catalyst, 2.4 mmol·L?1 CaO2, pH 7.0,22 ± 2 ℃ 95.4% /120 min Fifth cycles/
75.6%Zn-Fe-CNTs[50] Sulfamethoxazole 25 mg?L?1 0.6 g?L?1 catalyst,400 mL·min?1 O2,pH 1.5, 25 ℃ 100%/10 min,51.3% TOC/10 min NiFe2O4-MWCNT[12] Sulfamethoxazole 5 mg?L?1 0.025 g?L?1 catalyst,1 μL·mL?1 H2O2,100 W
Mercury lamp, 25 ℃100%/120 min,68% TOC/120 min Fifth cycles/
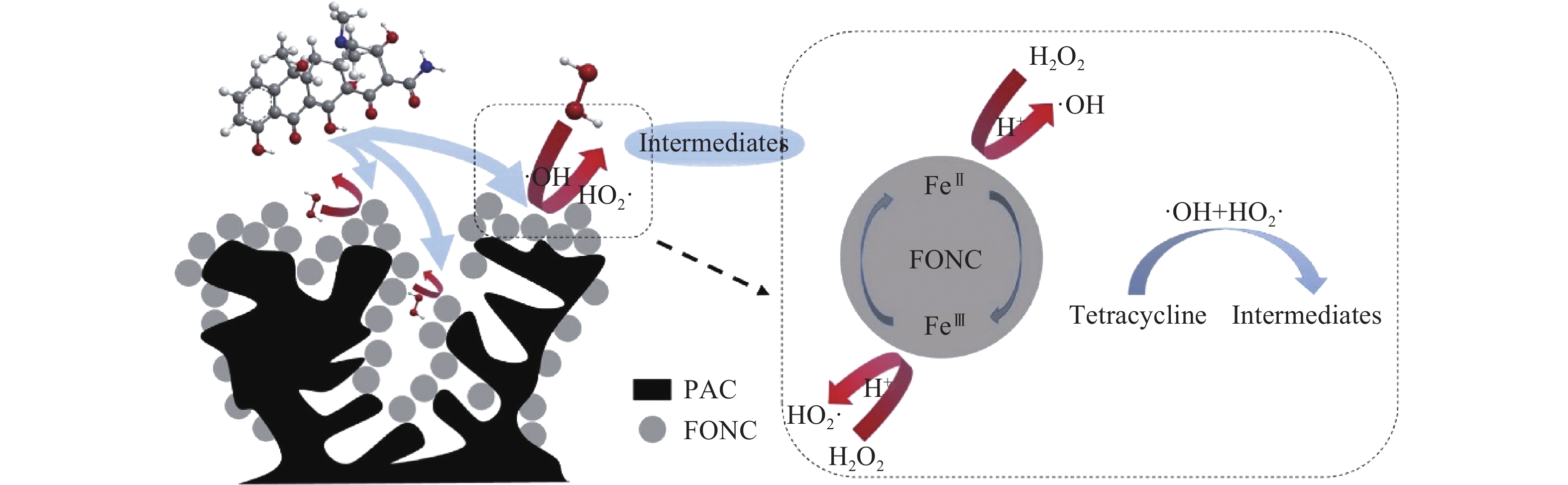
80%C@FONC[13] Tetracycline 0.015% 0.5 g·L?1 catalyst, 5.0 mmol·L?1 H2O2, pH 3.0 97.9%/180 min,52.7% TOC/180 min Ninth cycles/
85.8%ZIF-8/MnFe2O4[16] Tetracycline 20 mg?L?1 0.3 g?L?1 catalyst, 50 mmol·L?1 H2O2, 300 W Xenon lamp 92%/90 min Fifth cycle/
82.5%nZVI/MIL-101(Cr)[17] Tetracycline 200 mg?L?1 0.20 g?L?1 catalyst,50 mmol·L?1 H2O2, 25 ℃ 93%/120 min Fifth cycle/
87.34%表 2 外場輔助的類型及其優勢
Table 2. Types and advantages of outfield assistance
Types Mechanism and advantages Light assisted[23-26] 1) Photolysis of hydrogen peroxide increases the production of hydroxyl radicals;
2) Rapid regeneration of ferrous ion from iron complex under illumination;
3) Semiconductor materials produce photogenerated electron / hole pairs under illuminationElectrical assisted[27-28] 1) Hydrogen peroxide generated in situ by electrolysis;
2) Regeneration of ferrous ion by cathodic reduction;
3) Suitable for wide pH rangeUltrasound assisted[31-32] 1) More hydroxyl radicals are produced in solution by acoustic cavitation;
2) Promote the regeneration of ferrous ions;
3) Production of hydroxyl radical and hydrogen peroxide by ultrasonic radiation;
4) Improve interfacial mass transferMicrowave assisted[29-30] 1) Generate a large number of “hot spots” to accelerate the reduction of iron ions to ferrous ions;
2) Promote the decomposition of hydrogen peroxide to generate active free radicals;
3) Improve interfacial mass transfer259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Calvete M J F, Piccirillo G, Vinagreiro C S, et al. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coordin Chem Rev, 2019, 395: 63 doi: 10.1016/j.ccr.2019.05.004 [2] Brown E D, Wright G D. Antibacterial drug discovery in the resistance era. Nature, 2016, 529(7586): 336 doi: 10.1038/nature17042 [3] Gensollen T, Iyer S S, Kasper D L, et al. Antibiotic use and its consequences for the normal microbiome. Science, 2016, 352(6285): 539 doi: 10.1126/science.aad9378 [4] Aydin S, Ince B, Ince O. Assessment of anaerobic bacterial diversity and its effects on anaerobic system stability and the occurrence of antibiotic resistance genes. Bioresour Technol, 2016, 207: 332 doi: 10.1016/j.biortech.2016.01.080 [5] Ahmed M B, Zhou J L, Ngo H H, et al. Adsorptive removal of antibiotics from water and wastewater: progress and challenges. Scie Total Environ, 2015, 532: 112 doi: 10.1016/j.scitotenv.2015.05.130 [6] Leng L J, Wei L, Xiong Q, et al. Use of microalgae based technology for the removal of antibiotics from wastewater: a review. Chemosphere, 2020, 238: 124680 doi: 10.1016/j.chemosphere.2019.124680 [7] Cuerda-Correa E M, Alexandre-Franco M F, Fernández-González C. Advanced oxidation processes for the removal of antibiotics from water: an overview. Water, 2020, 12(1): 102 [8] Wang J L, Xu L J. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol, 2012, 42(3): 251 doi: 10.1080/10643389.2010.507698 [9] Yu X D, Lin X C, Feng W, et al. Effective removal of tetracycline by using bio-templated synthesis of TiO2/Fe3O4 heterojunctions as a UV–fenton catalyst. Catal Lett, 2019, 149(2): 552 doi: 10.1007/s10562-018-2544-8 [10] Guo T, Wang K, Zhang G K, et al. A novel α-Fe2O3@g-C3N4 catalyst: synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl Surf Sci, 2019, 469: 331 doi: 10.1016/j.apsusc.2018.10.183 [11] Qin Y X, Li G Y, Gao Y P, et al. Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: a critical review. Water Res, 2018, 137: 130 doi: 10.1016/j.watres.2018.03.012 [12] Nawaz M, Shahzad A, Tahir K, et al. Photo-Fenton reaction for the degradation of sulfamethoxazole using a multi-walled carbon nanotube-NiFe2O4 composite. Chem Eng J, 2020, 382: 123053 doi: 10.1016/j.cej.2019.123053 [13] Zhou J H, Ma F, Guo H J, et al. Activate hydrogen peroxide for efficient tetracycline degradation via a facile assembled carbon-based composite: synergism of powdered activated carbon and ferroferric oxide nanocatalyst. Appl Catal B Environ, 2020, 269: 118784 doi: 10.1016/j.apcatb.2020.118784 [14] Cheng M, Lai C, Liu Y, et al. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord Chem Rev, 2018, 368: 80 doi: 10.1016/j.ccr.2018.04.012 [15] Zhu G W, Wang S, Yu Z C, et al. Application of Fe-MOFs in advanced oxidation processes. Res Chem Intermed, 2019, 45(7): 3777 doi: 10.1007/s11164-019-03820-5 [16] Wang Z H, Lai C, Qin L, et al. ZIF-8-modified MnFe2O4 with high crystallinity and superior photo-Fenton catalytic activity by Zn?O?Fe structure for TC degradation. Chem Eng J, 2020, 392: 124851 doi: 10.1016/j.cej.2020.124851 [17] Hou X H, Shi J D, Wang N N, et al. Removal of antibiotic tetracycline by metal-organic framework MIL-101(Cr) loaded nano zero-valent iron. J Mol Liq, 2020, 313: 113512 doi: 10.1016/j.molliq.2020.113512 [18] Du W, Xu Q, Jin D Q, et al. Visible-light-induced photo-Fenton process for the facile degradation of metronidazole by Fe/Si codoped TiO2. RSC Adv, 2018, 8(70): 40022 doi: 10.1039/C8RA08114J [19] Hu Y, Chen K, Li Y L, et al. Morphology-tunable WMoO nanowire catalysts for the extremely efficient elimination of tetracycline: kinetics, mechanisms and intermediates. Nanoscale, 2019, 11(3): 1047 doi: 10.1039/C8NR08162J [20] Nguyen X S, Zhang G K, Yang X F. Mesocrystalline Zn-doped Fe3O4 hollow submicrospheres: Formation mechanism and enhanced photo-Fenton catalytic performance. ACS Appl Mater Interfaces, 2017, 9(10): 8900 doi: 10.1021/acsami.6b16839 [21] Sharma R, Bansal S, Singhal S. Augmenting the catalytic activity of CoFe2O4 by substituting rare earth cations into the spinel structure. RSC Adv, 2016, 6(75): 71676 doi: 10.1039/C6RA14325C [22] Vinosha P A, Xavier B, Krishnan S, et al. Investigation on zinc substituted highly porous improved catalytic activity of NiFe2O4 nanocrystal by co-precipitation method. Mater Res Bull, 2018, 101: 190 doi: 10.1016/j.materresbull.2018.01.026 [23] Casbeer E, Sharma V K, Li X Z. Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol, 2012, 87: 1 doi: 10.1016/j.seppur.2011.11.034 [24] Li Y J, Zhang B J, Liu X L, et al. Ferrocene-catalyzed heterogeneous Fenton-like degradation mechanisms and pathways of antibiotics under simulated sunlight: a case study of sulfamethoxazole. J Hazard Mater, 2018, 353: 26 doi: 10.1016/j.jhazmat.2018.02.034 [25] Xue Z H, Wang T, Chen B D, et al. Degradation of tetracycline with BiFeO3 prepared by a simple hydrothermal method. Materials, 2015, 8(9): 6360 doi: 10.3390/ma8095310 [26] Zhu G P, Yu X D, Xie F, et al. Ultraviolet light assisted heterogeneous Fenton degradation of tetracycline based on polyhedral Fe3O4 nanoparticles with exposed high-energy {110} facets. Appl Surf Sci, 2019, 485: 496 doi: 10.1016/j.apsusc.2019.04.239 [27] Rahmatinia Z, Rahmatinia M. Removal of the metronidazole from aqueous solution by heterogeneous electro-Fenton process using nano-Fe3O4. Data Brief, 2018, 19: 2139 doi: 10.1016/j.dib.2018.06.118 [28] ?zcan A, ?zcan A A, Demirci Y, et al. Preparation of Fe2O3 modified kaolin and application in heterogeneous electro-catalytic oxidation of enoxacin. Appl Catal B Environ, 2017, 200: 361 doi: 10.1016/j.apcatb.2016.07.018 [29] Zhu Y P, Zhu R L, Xi Y F, et al. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: a review. Appl Catal B Environ, 2019, 255: 117739 doi: 10.1016/j.apcatb.2019.05.041 [30] Qi Y, Mei Y Q, Li J Q, et al. Highly efficient microwave-assisted Fenton degradation of metacycline using pine-needle-like CuCo2O4 nanocatalyst. Chem Eng J, 2019, 373: 1158 doi: 10.1016/j.cej.2019.05.097 [31] Hou L W, Wang L G, Royer S, et al. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J Hazard Mater, 2016, 302: 458 doi: 10.1016/j.jhazmat.2015.09.033 [32] Ma X Y, Cheng Y Q, Ge Y J, et al. Ultrasound-enhanced nanosized zero-valent copper activation of hydrogen peroxide for the degradation of norfloxacin. Ultrason Sonochem, 2018, 40: 763 doi: 10.1016/j.ultsonch.2017.08.025 [33] Ding D H, Liu C, Ji Y F, et al. Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: Identification of radicals and degradation pathway. Chem Eng J, 2017, 308: 330 doi: 10.1016/j.cej.2016.09.077 [34] Pulicharla R, Drouinaud R, Brar S K, et al. Activation of persulfate by homogeneous and heterogeneous iron catalyst to degrade chlortetracycline in aqueous solution. Chemosphere, 2018, 207: 543 doi: 10.1016/j.chemosphere.2018.05.134 [35] Wang A Q, Chen Z, Zheng Z K, et al. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem Eng J, 2020, 379: 122340 doi: 10.1016/j.cej.2019.122340 [36] Huang M J, Zhou T, Wu X H, et al. Distinguishing homogeneous-heterogeneous degradation of norfloxacin in a photochemical Fenton-like system (Fe3O4 /UV/oxalate) and the interfacial reaction mechanism. Water Res, 2017, 119: 47 doi: 10.1016/j.watres.2017.03.008 [37] Wang Y, Liang J B, Liao X D, et al. Photodegradation of sulfadiazine by goethite?oxalate suspension under UV light irradiation. Ind Eng Chem Res, 2010, 49(8): 3527 doi: 10.1021/ie9014974 [38] Pan Y W, Zhou M H, Wang Q, et al. EDTA, oxalate, and phosphate ions enhanced reactive oxygen species generation and sulfamethazine removal by zero-valent iron. J Hazard Mater, 2020, 391: 122210 doi: 10.1016/j.jhazmat.2020.122210 [39] Neyens E, Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater, 2003, 98(1-3): 33 doi: 10.1016/S0304-3894(02)00282-0 [40] Pliego G, Zazo J A, Garcia-Mu?oz P, et al. Trends in the intensification of the Fenton process for wastewater treatment: an overview. Crit Rev Environ Sci Technol, 2015, 45(24): 2611 doi: 10.1080/10643389.2015.1025646 [41] Wang N N, Zheng T, Zhang G S, et al. A review on Fenton-like processes for organic wastewater treatment. J Environ Chem Eng, 2016, 4(1): 762 doi: 10.1016/j.jece.2015.12.016 [42] Jain B, Singh A K, Kim H, et al. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ Chem Lett, 2018, 16(3): 947 doi: 10.1007/s10311-018-0738-3 [43] Nidheesh P V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review. RSC Adv, 2015, 5(51): 40552 doi: 10.1039/C5RA02023A [44] Munoz M, de Pedro Z M, Casas J A, et al. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation— —a review. Appl Catal B Environ, 2015, 176-177: 249 doi: 10.1016/j.apcatb.2015.04.003 [45] Zha S X, Cheng Y, Gao Y, et al. Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem Eng J, 2014, 255: 141 doi: 10.1016/j.cej.2014.06.057 [46] Gao J S, Wu S C, Han Y L, et al. 3D mesoporous CuFe2O4 as a catalyst for photo-Fenton removal of sulfonamide antibiotics at near neutral pH. J Colloid Interface Sci, 2018, 524: 409 doi: 10.1016/j.jcis.2018.03.112 [47] Wang G, Zhao D Y, Kou F Y, et al. Removal of norfloxacin by surface Fenton system (MnFe2O4/H2O2): Kinetics, mechanism and degradation pathway. Chem Eng J, 2018, 351: 747 doi: 10.1016/j.cej.2018.06.033 [48] Tang J T, Wang J L. MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation. Chem Eng J, 2019, 375: 122007 doi: 10.1016/j.cej.2019.122007 [49] Amina, Si X Y, Wu K, et al. Mechanistic insights into the reactive radicals-assisted degradation of sulfamethoxazole via calcium peroxide activation by manganese-incorporated iron oxide–graphene nanocomposite: Formation of radicals and degradation pathway. Chem Eng J, 2020, 384: 123360 doi: 10.1016/j.cej.2019.123360 [50] Liu Y, Fan Q, Wang J L. Zn-Fe-CNTs catalytic in situ generation of H2O2 for Fenton-like degradation of sulfamethoxazole. J Hazard Mater, 2018, 342: 166 doi: 10.1016/j.jhazmat.2017.08.016 [51] Gao Y J, Champagne P, Blair D, et al. Activated persulfate by iron-based materials used for refractory organics degradation: a review. Water Sci Technol, 2020, 81(5): 853 doi: 10.2166/wst.2020.190 [52] Liang C J, Guo Y Y. Mass transfer and chemical oxidation of naphthalene particles with zerovalent iron activated persulfate. Environ Sci Technol, 2010, 44(21): 8203 doi: 10.1021/es903411a [53] Liu Y, Guo H G, Zhang Y L, et al. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin. Chem Eng J, 2018, 343: 128 doi: 10.1016/j.cej.2018.02.125 [54] Xiao S, Cheng M, Zhong H, et al. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: a review. Chem Eng J, 2020, 384: 123265 doi: 10.1016/j.cej.2019.123265 [55] Wu D L, Liu Y X, Zhang Z Y, et al. Pyrite-enhanced degradation of chloramphenicol by low concentrations of H2O2. Water Sci Technol, 2015, 72(2): 180 doi: 10.2166/wst.2015.202 [56] Munoz M, Conde J, de Pedro Z M, et al. Antibiotics abatement in synthetic and real aqueous matrices by H2O2/natural magnetite. Catal Today, 2018, 313: 142 doi: 10.1016/j.cattod.2017.10.032 [57] Sun F W, Liu H B, Wang H L, et al. A novel discovery of a heterogeneous Fenton-like system based on natural siderite: A wide range of pH values from 3 to 9. Sci Total Environ, 2020, 698: 134293 doi: 10.1016/j.scitotenv.2019.134293 [58] Kamagate M, Assadi A A, Kone T, et al. Use of laterite as a sustainable catalyst for removal of fluoroquinolone antibiotics from contaminated water. Chemosphere, 2018, 195: 847 doi: 10.1016/j.chemosphere.2017.12.165 [59] Ayala-Durán S C, Hammer P, Nogueira R F P. Surface composition and catalytic activity of an iron mining residue for simultaneous degradation of sulfonamide antibiotics. Environ Sci Pollut Res, 2020, 27(2): 1710 doi: 10.1007/s11356-019-06662-1 [60] Gr?ssinger R, Duong G V, Sato-Turtelli R. The physics of magnetoelectric composites. J Magn Magn Mater, 2008, 320(14): 1972 doi: 10.1016/j.jmmm.2008.02.031 [61] Sharma R, Kumar V, Bansal S, et al. Assortment of magnetic nanospinels for activation of distinct inorganic oxidants in photo-Fenton’s process. J Mol Catal A Chem, 2015, 402: 53 doi: 10.1016/j.molcata.2015.03.009 [62] Amiri M, Eskandari K, Salavati-Niasari M. Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv Colloid Interface Sci, 2019, 271: 101982 doi: 10.1016/j.cis.2019.07.003 [63] Chaibakhsh N, Moradi-Shoeili Z. Enzyme mimetic activities of spinel substituted nanoferrites (MFe2O4): a review of synthesis, mechanism and potential applications. Mater Sci Eng C, 2019, 99: 1424 doi: 10.1016/j.msec.2019.02.086 [64] Han X, Yan Z K, Chen T, et al. Phase transformation and catalytic performance of metal-doped MgFe2O4 prepared from saprolite laterite. Chin J Eng, 2019, 41(5): 600韓星, 閆治開, 陳婷, 等. 從腐泥土型紅土鎳礦制備共摻雜MgFe2O4物相轉化規律及催化性能. 工程科學學報, 2019, 41(5):600 [65] Liu Y X, Chen T, Han X, et al. Copper doping effect on the preparation of efficient heterogeneous Fenton-like catalyst (Ni, Mg, Cu)Fe2O4 from nickel sulfide concentrate. Chin J Eng, https://doi.org/10.13374/j.issn2095-9389.2020.06.18.002劉雅賢, 陳婷, 韓星, 等. Cu摻雜對硫化鎳精礦制備高效異相類Fenton催化劑(Ni, Mg, Cu)Fe2O4的影響. 工程科學學報, https://doi.org/10.13374/j.issn2095-9389.2020.06.18.002 [66] Magnago L B, Rocha A K S, Pegoretti V C B, et al. NiFe2O4 synthesized from nickel recycled of spent Ni-MH batteries and their applications as a catalyst in a photo-Fenton process and as an electrochemical pseudocapacitor. Ionics, 2019, 25(5): 2361 doi: 10.1007/s11581-018-2623-2 [67] Cao Z B, Zhang J, Zhou J Z, et al. Electroplating sludge derived zinc-ferrite catalyst for the efficient photo-Fenton degradation of dye. J Environ Manage, 2017, 193: 146 doi: 10.1016/j.jenvman.2016.11.039 [68] Li Y, Chen D, Fan S S, et al. Enhanced visible light assisted Fenton-like degradation of dye via metal-doped zinc ferrite nanosphere prepared from metal-rich industrial wastewater. J Taiwan Inst Chem Eng, 2019, 96: 185 doi: 10.1016/j.jtice.2018.11.006 [69] Han X, Chen T, Liu Y X, et al. Novel efficient heterogeneous visible light assisted Fenton-like catalyst (Ni, Mg, Cu)Fe2O4 from nickel sulfide concentrate. Mater Lett, 2019, 253: 1 doi: 10.1016/j.matlet.2019.06.014 [70] Han X, Liu S Y, Huo X T, et al. Facile and large-scale fabrication of (Mg, Ni)(Fe, Al)2O4 heterogeneous photo-Fenton-like catalyst from saprolite laterite ore for effective removal of organic contaminants. J Hazard Mater, 2020, 392: 122295 doi: 10.1016/j.jhazmat.2020.122295 -




 下載:
下載: