Development of template methods for the preparation of porous photocatalysts of graphite-like carbon nitride
-
摘要: 氮化碳作為一種具有高催化性能的光催化劑,具有無毒無害,自然環境下穩定的性質,在水解制氫氣氧氣以及降解有機污染物領域得到了廣泛的關注. 其中類石墨相氮化碳(g-C3N4)因其特殊的片層結構而具有較高比表面積,常配合孔結構的構造,提供光生載流子及反應物質的運輸通道以及大量活性位點用于氧化還原反應,是具有高光電性能的一種光催化劑.制備該種催化劑孔結構的方法有硬模板法,軟模板法與非模板法,其中硬模板法需要在實驗后除去模板,軟模板法的模板會隨著高溫除去,非模板法的制備過程沒有模板的參與。本文根據近年文獻的整理,著重闡述和比較各制備方法的優劣,結合常用的修飾手段總結各制備方法的變化趨勢和發展方向,并對后續研究中制備方法的使用前景做出判斷.Abstract: As a metal-free photocatalyst with high catalytic performance, carbon nitride is non-toxic, harmless, and stable in the natural environment. Owing to its facile synthesis, stable physical and chemical properties, tunable structure, and suitable band gap, graphite-like carbon nitride (g-C3N4) plays an increasing role in the field of photocatalysis. It has attracted extensive attention in the fields of evolution of hydrogen and oxygen via water-splitting hydrolysis and in the degradation of organic pollutants. In particular, g-C3N4 is identified to have a high specific surface area (SSA) because of its special lamellar structure. Meanwhile, the abundant pores intrinsic in it are able to provide both transporting channels for photogenic carriers or reactive species and a large number of active sites for redox reactions. These merits endow it with high photoelectrical properties. The preparation methods of the pore structures of such catalyst include hard templates, soft templates, and non-template ones. The hard template method enables the preparation of regular pore structures but requires additional removal treatment. However, the soft templates can be decomposed during the high-temperature preparation of g-C3N4, which avoids the use of toxic reagents and consequently is harmless to the environment, and the template-free method does not involve any templates, which will simplify the experimental process from the aspect of sample preparation with reduced cost. In this paper, the advantages and disadvantages of various preparation methods were elaborated and compared based on the literature review in recent years. The developments and applications in the environmental and energy aspects were summarized by combining the commonly used modification methods, which provided the perceptions with respect to the development of metal-free g-C3N4-based photocatalysts in the future. Further, the photocatalytic mechanism was explained, and the four different precursors of g-C3N4 were compared. Finally, the ongoing outlook and perspectives will be covered in this review.
-
Key words:
- graphite-like carbon nitride /
- photocatalyst /
- porous structure /
- template method /
- template-free method
-
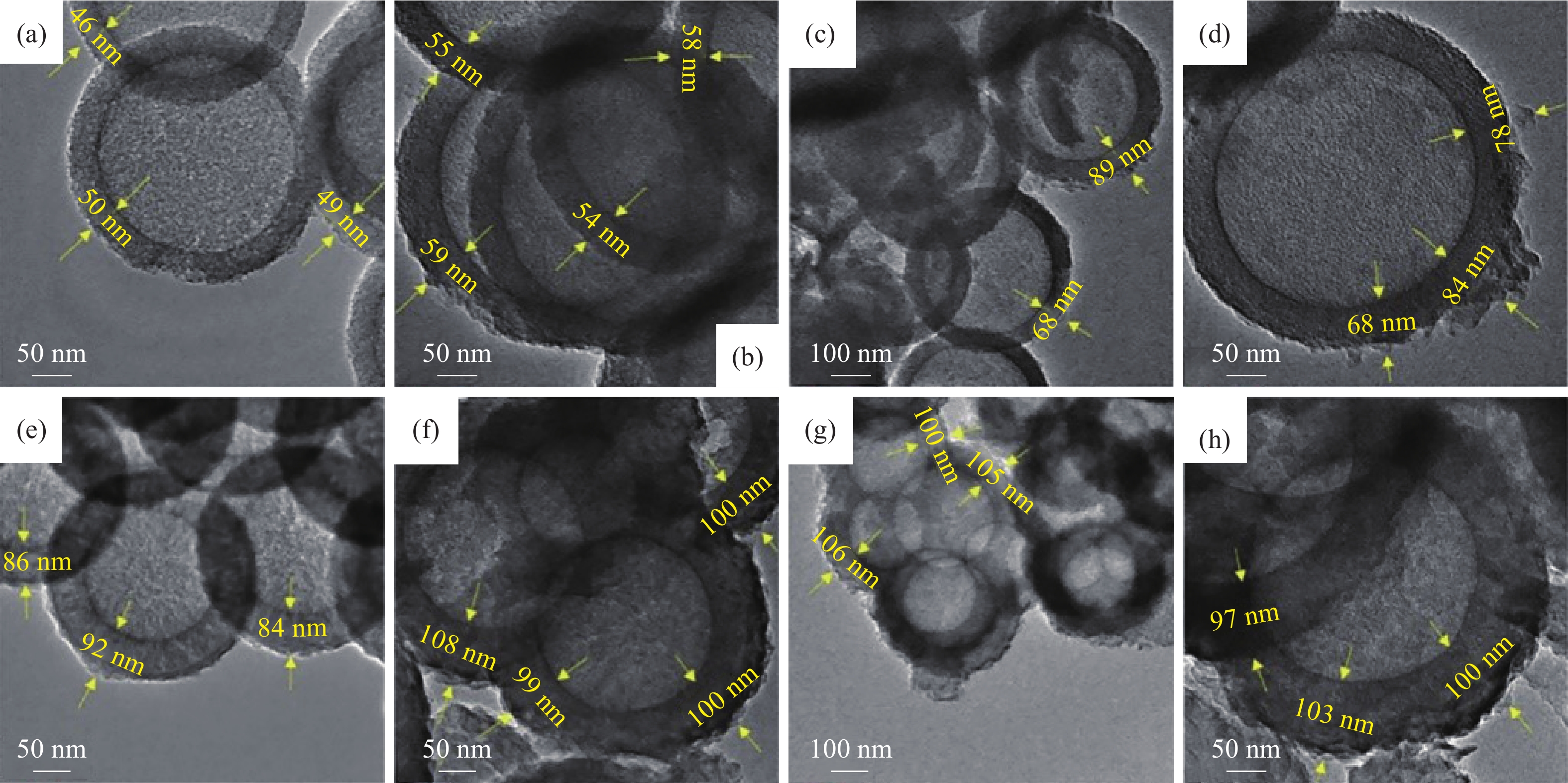
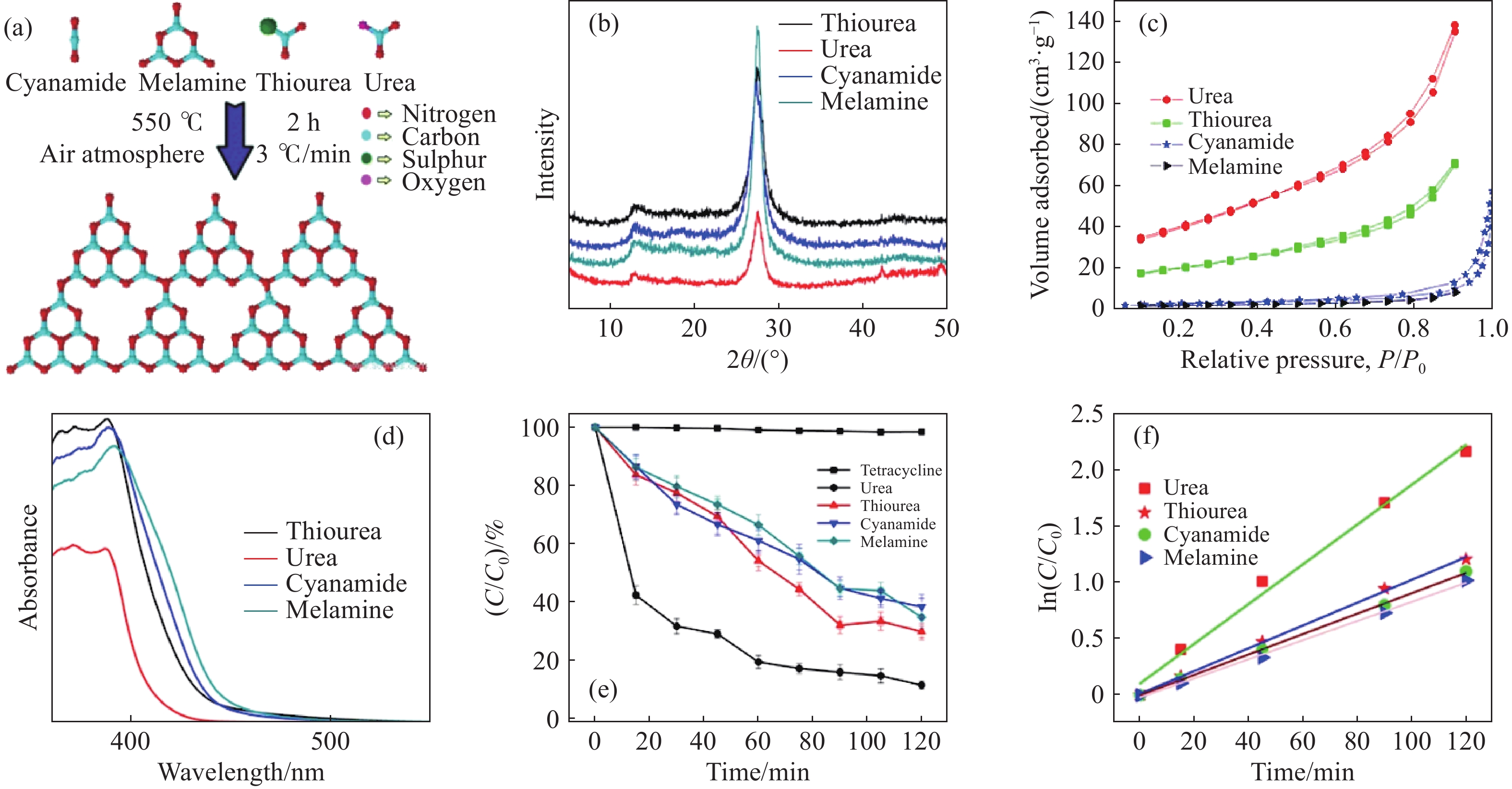
圖 4 (a)圖解說明由不同前體合成的聚合C3N4形成的示意圖;(b)由不同前體合成的C3N4的X射線衍射圖譜;(c)由不同前體合成的C3N4的N2吸附?解吸等溫線;(d)由不同前體合成的C3N4的UV-Vis吸收光譜;(e)使用由不同前體合成的C3N4降解TC的效率圖;(f)由不同前體合成的C3N4對TC的光降解速率[36]
Figure 4. (a) Schematic illustrating the formation of polymeric C3N4 synthesized from different precursors; (b) XRD pattern of C3N4 synthesized from different precursors; (c) nitrogen adsorption?desorption isotherms of C3N4 synthesized from different precursors; (d) UV-Vis absorption spectra of C3N4 synthesized from different precursors; (e) degradation pattern of TC using C3N4 synthesized from different precursors; (f) photodegradation rate of TC using C3N4 synthesized from different precursors [36]
表 1 不同模板法調控g-C3N4孔結構總括
Table 1. Summary of the different template methods in adjusting the pore structure of g-C3N4
Sample Reaction precursor, temperature, time, heating rate, & atmosphere Template type& requirement Removal reagents& requirement Specific surface
area/(m2?g?1)Pore volume/
(cm3?g?1)Average pore
size/nmRefs. Porous-C3N4 Dicyandiamide, 500 °C, 4.5 h 7?40 nm SiO2 hard template 20% HF, 4 h ~109 — ~20 [20] MCN1.0 Cyanamide, 550 °C, 4 h, 2 °C?min?1, N2 12 nm SiO2 nanosphere hard template 4.0 mol?L?1
NH4HF2, 24 h~190.7 ~0.52 ~10.9 [22] CLBM?SBA-15 Cyanamide, stirring at room temperature, 1 h, 550 °C, 6 h, air 9.5 nm SBA-15 hard template 4.0 mol?L?1
NH4HF2~145 ~0.43 ~44 [23] C3N4?MCF Melamine, 300 °C, 1 h, 600 °C, 2 h, Ar MCF hard template 15% HF, 12 h ~70 ~0.3 — [24] Bulk-g-C3N4 Dicyandiamide, 560 °C, 2 h, N2 12 nm PSB hard template NH4HF2 ~37 ~0.28 ~36 [25] TiO2 trapped g-C3N4 Dicyandiamide, 560 °C, 2 h, N2 12 nm PSB hard template NH4HF2 ~63 ~0.29 ~20 [25] OCS/gCN Melamine, 550 °C, 2 h, 2 °C?min?1, air 260?320 nm SiO2 nanosphere hard template HF ~105 ~0.123 ~260?320 [26] Meso-g-C3N4/WP/Meso-g-C3N4 Cyanamide, 550 °C, 4 h, 3 °C?min?1, N2 8?15 nm SiO2 hard template 0.5 mol?L?1 HF ~82 — ~8?15 [27] CN?MCF-0.4 Carbon tetrachloride, ethylenediamine,
90 °C, 6 h, 600 °C, 5 h, 3 °C?min?1, Ar35.7 nm MCF hard template 4.0 mol?L?1
NH4HF2~498 ~1.36 ~5.3 [29] mpg?C3N4-δ Dicyandiamide, 550 °C, 4 h,
2.3 °C?min?1, N212 nm SiO2 hard template 4.0 mol?L?1
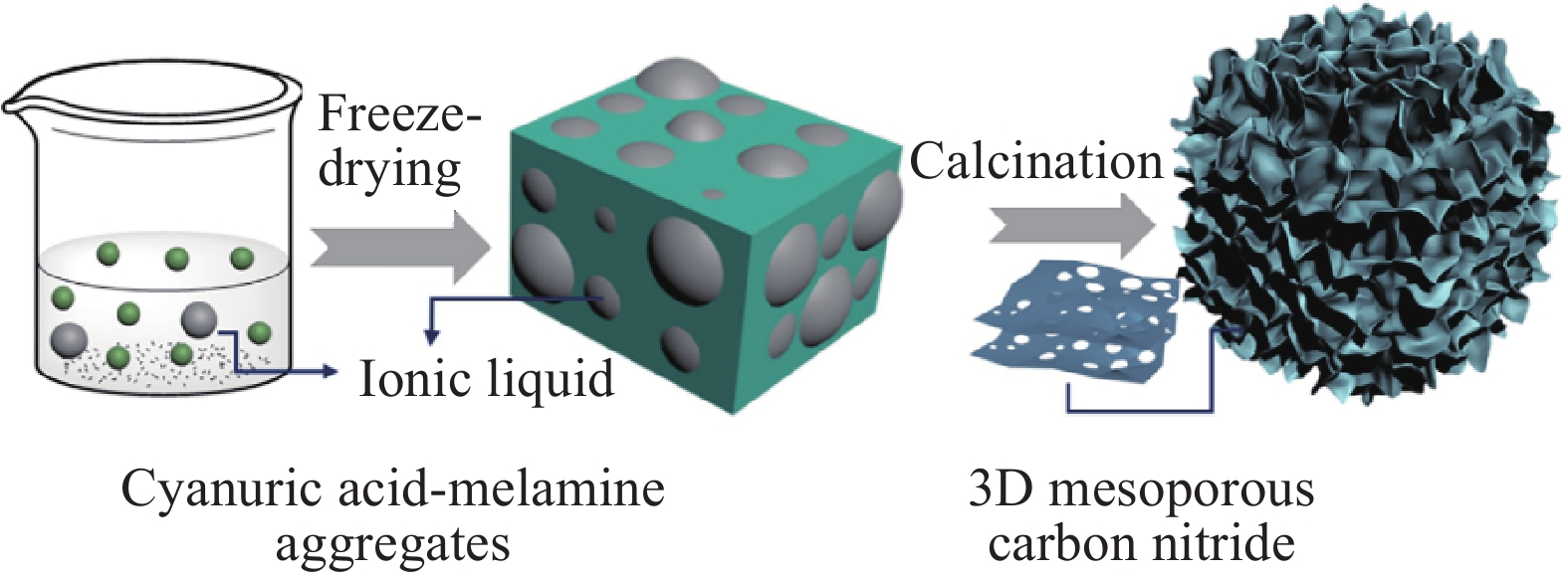
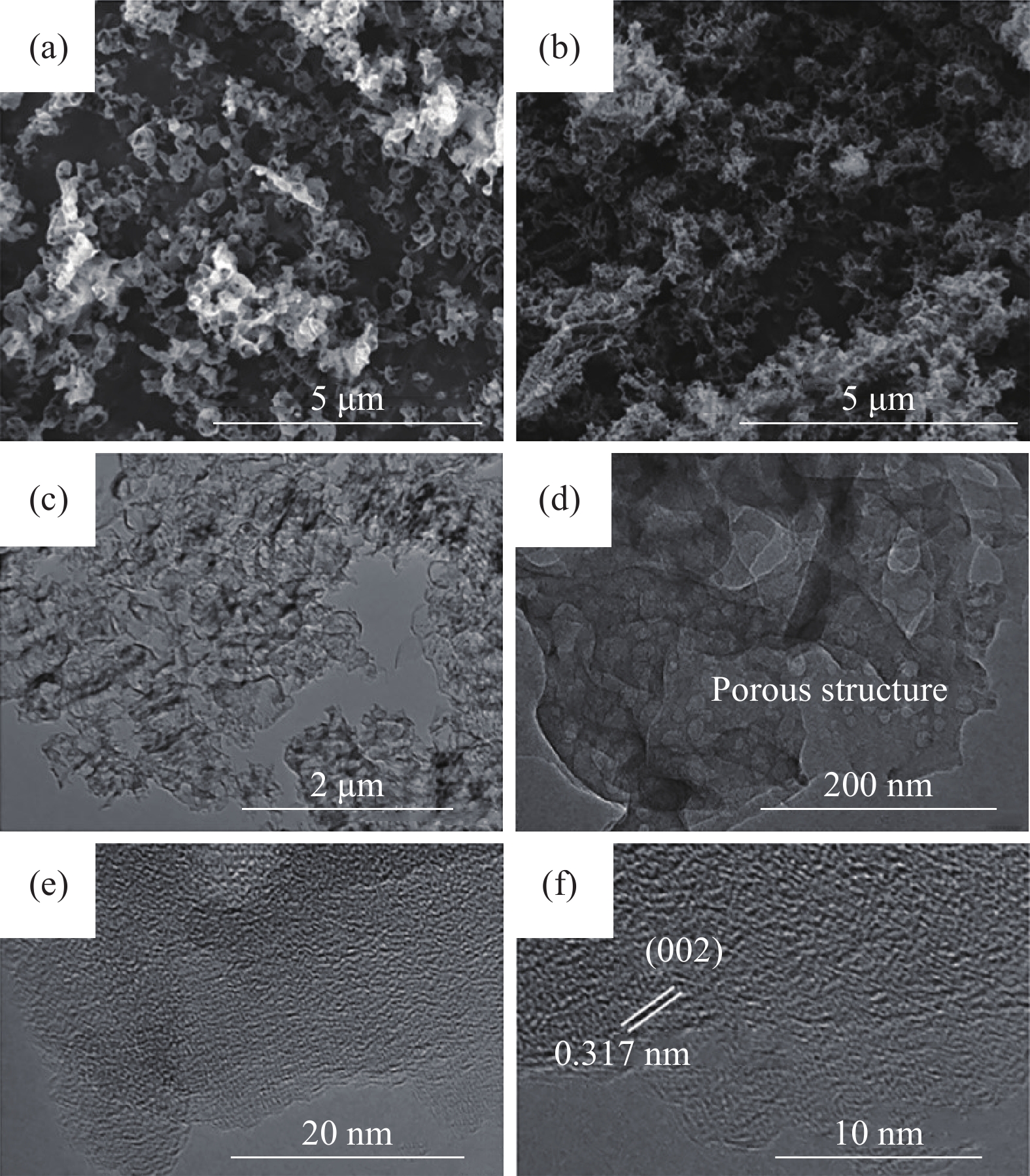
NH4HF2, 24 h~218.15 ~0.69 ~12.57 [30] PCNCs?ANTa Melamine, 550 °C, 2 h, 2 °C?min?1 6.7 nm ZnO hard template 4.0 mol?L?1 NaOH ~32 ~0.103 ~16.8 [31] g-C3N4 Melamine, 500 °C, 2 h, air P123 soft template ~90 — — [34] Bulk-g-C3N4 Melamine, 600 °C, 2 h, Ar P123 soft template ~90 — ~15 [34] g-C3N4/SnO2 Thiourea, SnCl4, 550 °C, 2 h, 10 °C?min?1 HCl, H2O soft template ~44.3 2.638 ~100?430 [35] C3N4@TiO2 Melamine, 400 °C, 2 h, N2, 2 h, air Melamine soft template ~44.7 ~0.11 ~10.7 [37] N-RGO Graphene oxide, melamine, 900 °C,
30 min, 5 °C?min?1Ice soft template ~190 ~0.99 ~20?200 [38] CNF-0.005 Melamine, 550 °C, 4 h, 3 °C?min?1, N2 Cyanuric acid-melamine supramolcular aggregates & ionic liquid soft template ~381 ~0.85 ~15 [39] PCNM Melamine, urea, 550 °C, 4 h, N2 Melamine soft template ~78 ~0.76 — [40] g-C3N4 ultrathin nanosheet Melamine, glutaraldehyde, 800 °C,
2 h, ArCyanuric acid soft template ~84 — ~3 [48] g-C3N4 bundles Melamine, 500 °C, 4 h, N2 PEG-PPG-PEG soft template ~40.974 — ~100 [49] g-C3N4 beads Dicyandiamide, 530?600 °C, 2 h, N2 PSB soft template ~58 ~0.15 ~30?90 [50] P-C3N4 Melamine, 550 °C, 4 h Free ~40.89 ~0.2 — [43] 2MCN/2UCN Melamine, urea, 550 °C, 1.5 h,
5 °C?min?1, N2Free ~41 ~0.24 ~23 [44] 5% La/g-CNT Melamine, 550 °C, 2 h, air Free ~4 ~0.0623 ~2.8 [45] CN/Fe-1 Urea, 600 °C, 4 h, 5 °C?min?1 Free ~48.19 — — [46] g-C3N4 NS/TMC Melamine, 550 °C, 3 h, 2.3 °C?min?1, air Free ~57.4 — — [47] g-C3N4/Ag3PO4 Urea, 550 °C, 4 h, Ar Free ~20.84 ~0.083 ~16 [51] Brookite/anatase TiO2/g-C3N4 Hexadecylamine, 550 °C, 4 h,
3 °C?min?1, N2Free ~37 ~0.2 ~18 [52] g-C3N4 nanosheet Melamine, 550 °C, 4 h, 500 °C, 2 h, Ar Free ~190.1 ~0.61 ~5?25 [53] Fe2O3(6.6)/CNSb Thiourea, 535 °C, 3 h, 3 °C?min?1, air Free ~33.5 ~0.195 ~25 [54] g-C3N4/MoS2 Thiourea, 500 °C, 2 h, 2 °C?min?1 Free ~45 — — [55] 259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Tian H F, Song L M. Recent advances of g-C3N4 visible light photocatalysts. J Tianjin Polytech Univ, 2012, 31(6): 55 doi: 10.3969/j.issn.1671-024X.2012.06.014田海鋒, 宋立民. g-C3N4光催化劑研究進展. 天津工業大學學報, 2012, 31(6):55 doi: 10.3969/j.issn.1671-024X.2012.06.014 [2] He F, Wang Z X, Li Y X, et al. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl Catal B, 2020, 269: 118828 doi: 10.1016/j.apcatb.2020.118828 [3] Yan Q Y, Zhao C C, Zhang L, et al. Facile two-step synthesis of porous carbon nitride with enhanced photocatalytic activity using a soft template. ACS Sustainable Chem Eng, 2019, 7(4): 3866 doi: 10.1021/acssuschemeng.8b04873 [4] Babu B, Shim J, Kadam A N, et al. Modification of porous g-C3N4 nanosheets for enhanced photocatalytic activity: In-situ synthesis and optimization of NH4Cl quantity. Catal Commun, 2019, 124: 123 doi: 10.1016/j.catcom.2019.01.009 [5] Lu J, Wang Y, Huang J F, et al. One-step synthesis of g-C3N4 hierarchical porous structure nanosheets with dramatic ultraviolet light photocatalytic activity. Mater Sci Eng B, 2016, 214: 19 doi: 10.1016/j.mseb.2016.08.003 [6] Han D Y, Liu J, Cai H, et al. High-yield and low-cost method to synthesize large-area porous g-C3N4 nanosheets with improved photocatalytic activity for gaseous nitric oxide and 2-propanol photodegradation. Appl Surf Sci, 2019, 464: 577 doi: 10.1016/j.apsusc.2018.09.108 [7] Li Y, Zhang D N, Fan J J, et al. Highly crystalline carbon nitride hollow spheres with enhanced photocatalytic performance. Chin J Catal, 2021, 42: 627 [8] Yang Z X, Chu D L, Jia G R, et al. Significantly narrowed bandgap and enhanced charge separation in porous, nitrogen-vacancy red g-C3N4 for visible light photocatalytic H2 production. Appl Surf Sci, 2020, 504: 144407 doi: 10.1016/j.apsusc.2019.144407 [9] Wu X H, Ma H Q, Zhong W, et al. Porous crystalline g-C3N4: Bifunctional NaHCO3 template-mediated synthesis and improved photocatalytic H2-evolution rate. Appl Catal B, 2020, 271: 118899 doi: 10.1016/j.apcatb.2020.118899 [10] Chen J Q, Lin W T, Xie L Y, et al. Templated fabrication of graphitic carbon nitride with ordered mesoporous nanostructures for high-efficient photocatalytic bacterial inactivation under visible light irradiation. J Nanomater, 2019, 2019: 3242136 [11] Chen W, Liu M, Wei S J, et al. Solid-state synthesis of ultrathin MoS2 as a cocatalyst on mesoporous g-C3N4 for excellent enhancement of visible light photoactivity. J Alloys Compd, 2020, 836: 155401 doi: 10.1016/j.jallcom.2020.155401 [12] Wu M, Yan J M, Zhang X W, et al. Synthesis of g-C3N4 with heating acetic acid treated melamine and its photocatalytic activity for hydrogen evolution. Appl Surf Sci, 2015, 354: 196 doi: 10.1016/j.apsusc.2015.01.132 [13] Xiao J D, Xie Y B, Li C H, et al. Enhanced hole-dominated photocatalytic activity of doughnut-like porous g-C3N4 driven by down-shifted valance band maximum. Catal Today, 2018, 307: 147 doi: 10.1016/j.cattod.2017.02.024 [14] Li Y Y, Zhu S L, Liang Y Q, et al. One-step synthesis of Mo and S co-doped porous g-C3N4 nanosheets for efficient visible-light photocatalytic hydrogen evolution. Appl Surf Sci, 2021, 536: 147743 [15] Zhang M, Xu J, Zong R L, et al. Enhancement of visible light photocatalytic activities via porous structure of g-C3N4. Appl Catal B, 2014, 147: 229 doi: 10.1016/j.apcatb.2013.09.002 [16] He F, Chen G, Zhou Y S, et al. The facile synthesis of mesoporous g-C3N4 with highly enhanced photocatalytic H2 evolution performance. Chem Commun, 2015, 51(90): 16244 doi: 10.1039/C5CC06713H [17] Li X B, Xiong J, Gao X M, et al. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J Alloys Compd, 2019, 802: 196 doi: 10.1016/j.jallcom.2019.06.185 [18] Chen D, Yang J, Ding H. Synthesis of nanoporous carbon nitride using calcium carbonate as templates with enhanced visible-light photocatalytic activity. Appl Surf Scie, 2017, 391: 384 [19] Wang W, Fang J J, Chen H. Nano-confined g-C3N4 in mesoporous SiO2 with improved quantum size effect and tunable structure for photocatalytic tetracycline antibiotic degradation. J Alloys Compd, 2020, 819: 153064 doi: 10.1016/j.jallcom.2019.153064 [20] Li Y P, Qu W P, Huang L Y, et al. Porous-C3N4 with high ability for selective adsorption and photodegradation of dyes under visible-light. J Inorg Organomet Polym Mater, 2017, 27(6): 1674 doi: 10.1007/s10904-017-0629-2 [21] Liu H J, Wu H N, Lü J, et al. SBA-15 templated mesoporous graphitic C3N4 for remarkably enhanced photocatalytic degradation of organic pollutants under visible light. Nano, 2019, 14(11): 1950136 doi: 10.1142/S1793292019501364 [22] Wang J J, Wang Y, Wang W, et al. Tunable mesoporous g-C3N4 nanosheets as a metal-free catalyst for enhanced visible-light-driven photocatalytic reduction of U(VI). Chem Eng J, 2020, 383: 123193 doi: 10.1016/j.cej.2019.123193 [23] Zhao H M, Di C M, Wang L, et al. Synthesis of mesoporous graphitic C3N4 using cross-linked bimodal mesoporous SBA-15 as a hard template. Microporous Mesoporous Mater, 2015, 208: 98 doi: 10.1016/j.micromeso.2015.01.047 [24] Ovcharov M, Shcherban N, Filonenko S, et al. Hard template synthesis of porous carbon nitride materials with improved efficiency for photocatalytic CO2 utilization. Mater Sci Eng B, 2015, 202: 1 doi: 10.1016/j.mseb.2015.08.003 [25] Wu W B, Li X, Ruan Z H, et al. Fabrication of a TiO2 trapped meso/macroporous g-C3N4 heterojunction photocatalyst and understanding its enhanced photocatalytic activity based on optical simulation analysis. Inorg Chem Front, 2018, 5(2): 481 doi: 10.1039/C7QI00751E [26] Baca M, Dworczak M, Aleksandrzak M, et al. Mesoporous carbon/graphitic carbon nitride spheres for photocatalytic H2 evolution under solar light irradiation. Int J Hydrogen Energy, 2020, 45(15): 8618 doi: 10.1016/j.ijhydene.2020.01.105 [27] Yang Z K, Xing Z P, Feng Q M, et al. Sandwich-like mesoporous graphite-like carbon nitride(Meso-g-C3N4)/WP/Meso-g-C3N4 laminated heterojunctions solar-driven photocatalysts. J Colloid Interface Sci, 2020, 568: 255 doi: 10.1016/j.jcis.2020.02.060 [28] Chen Z X, Zheng B Y, Li X X, et al. Progress in the preparation of nanomaterials employing template method. Chem Ind Eng Prog, 2010, 29(1): 94陳彰旭, 鄭炳云, 李先學, 等. 模板法制備納米材料研究進展. 化工進展, 2010, 29(1):94 [29] Xu J, Shen K, Xue B, et al. Synthesis of three-dimensional mesostructured graphitic carbon nitride materials and their application as heterogeneous catalysts for knoevenagel condensation reactions. Catal Lett, 2013, 143(6): 600 doi: 10.1007/s10562-013-0994-6 [30] Zhang S, Hu C, Ji H H, et al. Facile synthesis of nitrogen-deficient mesoporous graphitic carbon nitride for highly efficient photocatalytic performance. Appl Surf Sci, 2019, 478: 304 doi: 10.1016/j.apsusc.2019.01.270 [31] Tang J, Zhang Q T, Liu Y T, et al. The photocatalytic redox properties of polymeric carbon nitride nanocages(PCNCs) with mesoporous hollow spherical structures prepared by a ZnO-template method. Microporous Mesoporous Mater, 2020, 292: 109639 doi: 10.1016/j.micromeso.2019.109639 [32] Iqbal W, Wang L Z, Tan X J, et al. One-step in situ green template mediated porous graphitic carbon nitride for efficient visible light photocatalytic activity. J Environ Chem Eng, 2017, 5(4): 3500 doi: 10.1016/j.jece.2017.07.011 [33] Fei B, Tang Y W, Wang X Y, et al. One-pot synthesis of porous g-C3N4 nanomaterials with different morphologies and their superior photocatalytic performance. Mater Res Bull, 2018, 102: 209 doi: 10.1016/j.materresbull.2018.02.041 [34] Yan H J. Soft-templating synthesis of mesoporous graphitic carbon nitride with enhanced photocatalytic H2 evolution under visible light. Chem Commun, 2012, 48(28): 3430 doi: 10.1039/c2cc00001f [35] Chen Y Z, Li W H, Jiang D J, et al. Facile synthesis of bimodal macroporous g-C3N4/SnO2 nanohybrids with enhanced photocatalytic activity. Chin Sci Bull, 2019, 64(1): 44 [36] Panneri S, Ganguly P, Nair B N, et al. Role of precursors on the photophysical properties of carbon nitride and its application for antibiotic degradation. Environ Sci Pollut Res, 2017, 24(9): 8609 doi: 10.1007/s11356-017-8538-z [37] Li F X, Xiao X D, Zhao C, et al. TiO2-on-C3N4 double-shell microtubes: In-situ fabricated heterostructures toward enhanced photocatalytic hydrogen evolution. J Colloid Interface Sci, 2020, 572: 22 doi: 10.1016/j.jcis.2020.03.071 [38] Kota M, Yu X, Yeon S H, et al. Ice-templated three dimensional nitrogen doped graphene for enhanced supercapacitor performance. J Power Sources, 2016, 303: 372 doi: 10.1016/j.jpowsour.2015.11.006 [39] Zhao S, Fang J S, Wang Y Y, et al. Construction of three-dimensional mesoporous carbon nitride with high surface area for efficient visible-light-driven hydrogen evolution. J Colloid Interface Sci, 2020, 561: 601 doi: 10.1016/j.jcis.2019.11.035 [40] Liang Q H, Li Z, Yu X L, et al. Macroscopic 3D porous graphitic carbon nitride monolith for enhanced photocatalytic hydrogen evolution. Adv Mater, 2015, 27(31): 4634 doi: 10.1002/adma.201502057 [41] Azimi E B, Badiei A, Sabr M H, et al. A template-free method to synthesize porous G-C3N4 with efficient visible light photodegradation of organic pollutants in water. Adv Powder Technol, 2018, 29(11): 2785 doi: 10.1016/j.apt.2018.07.027 [42] She X J, Liu L, Ji H Y, et al. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl Catal B, 2016, 187: 144 doi: 10.1016/j.apcatb.2015.12.046 [43] Wang P Y, Guo C S, Hou S, et al. Template-free synthesis of bubble-like phosphorus-doped carbon nitride with enhanced visible-light photocatalytic activity. J Alloys Compd, 2018, 769: 503 doi: 10.1016/j.jallcom.2018.08.034 [44] Xu Q L, Ma D K, Yang S B, et al. Novel g-C3N4/g-C3N4 S-scheme isotype heterojunction for improved photocatalytic hydrogen generation. Appl Surf Sci, 2019, 495: 143555 doi: 10.1016/j.apsusc.2019.143555 [45] Muhammad A, Tahir M, Al-Shahrani S S, et al. Template free synthesis of graphitic carbon nitride nanotubes mediated by lanthanum (La/g-CNT) for selective photocatalytic CO2 reduction via dry reforming of methane (DRM) to fuels. Appl Surf Sci, 2020, 504: 144177 doi: 10.1016/j.apsusc.2019.144177 [46] Luo B, Song R, Geng J F, et al. Strengthened spatial charge separation over Z-scheme heterojunction photocatalyst for efficient photocatalytic H2 evolution. Appl Surf Sci, 2019, 475: 453 doi: 10.1016/j.apsusc.2018.12.285 [47] Elbanna O, Fujitsuka M, Majima T. g-C3N4/TiO2 mesocrystals composite for H2 evolution under visible-light irradiation and its charge carrier dynamics. ACS Appl Mater Interfaces, 2017, 9(40): 34844 doi: 10.1021/acsami.7b08548 [48] Zhao S, Zhang Y W, Zhou Y M, et al. Facile one-step synthesis of hollow mesoporous g-C3N4 spheres with ultrathin nanosheets for photoredox water splitting. Carbon, 2018, 126: 247 doi: 10.1016/j.carbon.2017.10.033 [49] Dai X H, Han Z W, Waterhouse G I N, et al. Ordered graphitic carbon nitride tubular bundles with efficient electron-hole separation and enhanced photocatalytic performance for hydrogen generation. Appl Catal A, 2018, 566: 200 doi: 10.1016/j.apcata.2018.09.001 [50] Li Y D, Jiang Y Q, Ruan Z H, et al. Simulation-guided synthesis of graphitic carbon nitride beads with 3D interconnected and continuous meso/macropore channels for enhanced light absorption and photo-catalytic performance. J Mater Chem A, 2017, 5: 21300 doi: 10.1039/C7TA06626K [51] Jiang D L, Zhu J J, Chen M, et al. Highly efficient heterojunction photocatalyst based on nanoporous g-C3N4 sheets modified by Ag3PO4 nanoparticles: Synthesis and enhanced photocatalytic activity. J Colloid Interface Sci, 2014, 417: 115 doi: 10.1016/j.jcis.2013.11.042 [52] Wei H, McMaster W A, Tan J Z Y, et al. Tricomponent brookite/anatase TiO2/g-C3N4 heterojunction in mesoporous hollow microspheres for enhanced visible-light photocatalysis. J Mater Chem A, 2018, 6(16): 7236 doi: 10.1039/C8TA00386F [53] Xu J, Wang Z P, Zhu Y F. Enhanced visible-light-driven photocatalytic disinfection performance and organic pollutant degradation activity of porous g-C3N4 nanosheets. ACS Appl Mater Interfaces, 2017, 9(33): 27727 doi: 10.1021/acsami.7b07657 [54] Jourshabani M, Shariatinia Z, Badiei A. High efficiency visible-light-driven Fe2O3?xSx/S-doped g-C3N4 heterojunction photocatalysts: Direct Z-scheme mechanism. J Mater Sci Technol, 2018, 34(9): 1511 doi: 10.1016/j.jmst.2017.12.020 [55] Qi Y R, Liang Q H, Lü R T, et al. Synthesis and photocatalytic activity of mesoporous g-C3N4/MoS2 hybrid catalysts. R Soc Open Sci, 2018, 5(5): 180187 doi: 10.1098/rsos.180187 -




 下載:
下載: