-
摘要: 相變材料的微膠囊化能解決相變材料在相變過程中的熔融滲出問題,提高相變材料的環境適應性、拓展其應用。本文主要對300 ℃以上的高溫相變微膠囊材料的制備及其應用進行闡述,主要論述了相變材料的分類,微膠囊的合成方法,以及高溫微膠囊的研究現狀。且通過研究表明,具有高熔點、高焓值的氟化物微膠囊是一種非常有應用前景的相變材料。Abstract: In today’s world, global problems such as a shortage of fossil fuel energy, environmental pollution, and global warming are becoming increasingly serious. For the development of human society, sustainability is particularly important. Energy is the basis for human survival and promotes the development of human society. However, rapid growth in population and the global economy has led to a significant increase in energy demand. At the same time, extensive use of fossil fuels has polluted the environment and led to a shortage of fossil energy. Currently, with the continuous increase in energy consumption and development of human society, there is a pressing need to develop energy storage technology. Latent heat storage, using phase change materials that play a vital role in the field of energy storage, has been widely accepted as an effective way to improve heat energy utilization. Phase change materials provide a type of thermal energy storage that can store a large amount of latent heat through physical phase change. This heat is then released in a controlled manner within a small temperature change based on thermal energy requirements. At present, phase change materials have important applications in aerospace, industrial and agricultural production, building materials, energy and power, textile materials, highway transportation, and engine technology. Most current research on phase change materials focuses on medium- and low-temperature materials, especially those materials whose phase change temperature is lower than 100 ℃. There is less research on high-temperature phase change materials owing to the encapsulation and corrosion of such materials. The problem of performance is difficult to solve, yet high temperature phase change materials are in urgent need in some extreme high temperature environments. High-temperature phase change materials (HTPCM) can control thermal energy under extremely high temperatures. They have important prospects for application in the fields of thermal protection and thermal management in high-temperature environments such as aerospace, solar energy, etc. The microencapsulation of phase change materials can solve the problem of melt exudation of these materials during the phase change process, improve the environmental adaptability of these materials, and expand their applications. This article mainly reviewed the preparation and application of HTPCM above 300 ℃. The classification of phase change materials, the method of synthesis of microcapsules, and the preparation of high temperature microcapsules were discussed. Through research, it is found that fluoride microcapsules, with their high melting point and enthalpy value, are a promising material in the field of HTPCMs.
-
Key words:
- phase change materials /
- high temperature /
- microcapsules /
- energy storage /
- thermal protection /
- thermal management
-
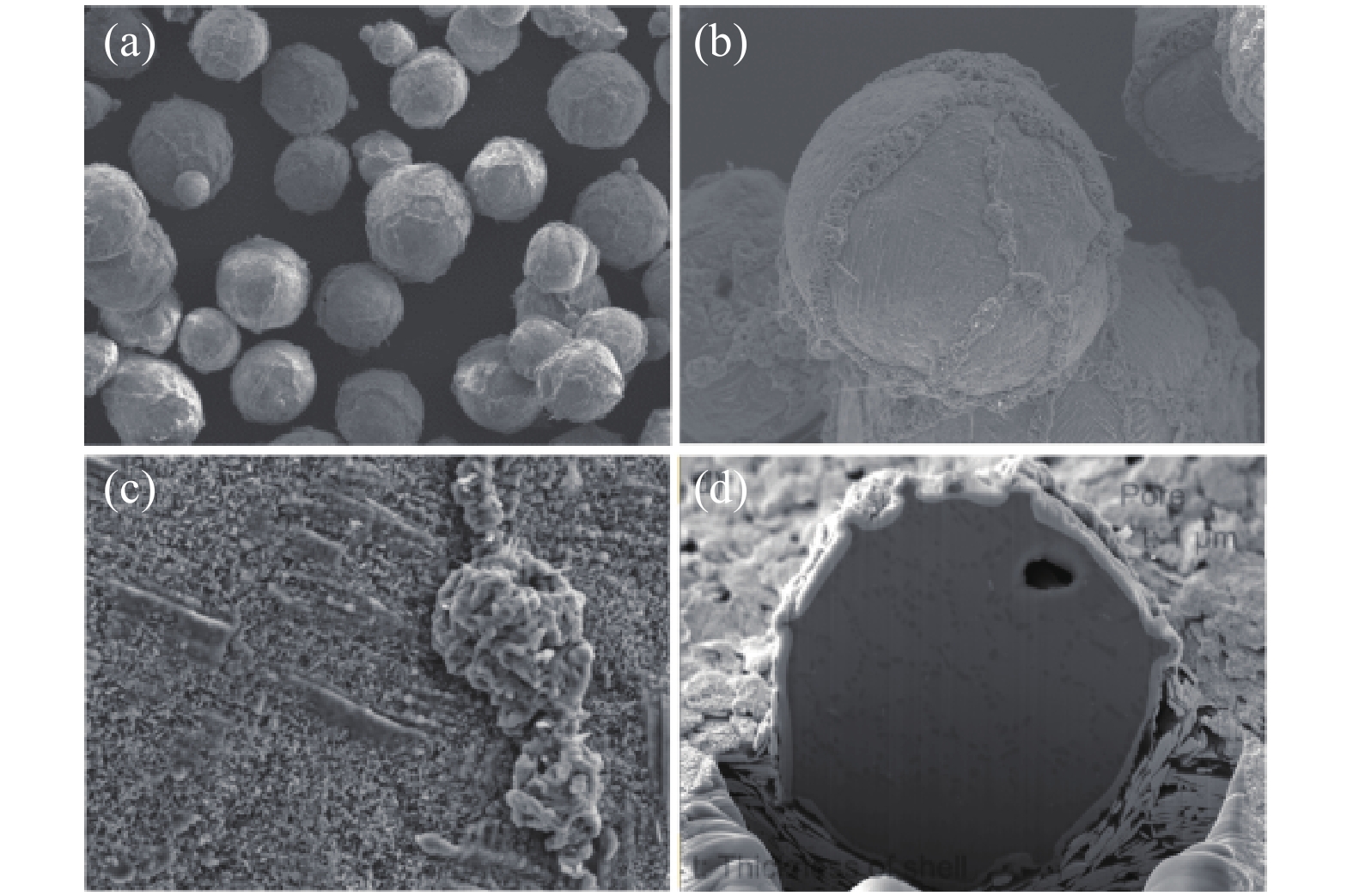
圖 5 加熱后的微膠囊的SEM照片。(a)將沒有GO防腐蝕防漏層的LiF @ PDA @ SiO2微膠囊從35 ℃加熱至1000 ℃;(b)將沒有SiO2耐熱強度層的LiF @ PDA @ GO微膠囊從35 ℃加熱到1000 ℃;(c)將LiF @ GO @ SiO2微囊從35 ℃加熱到1000 ℃;(d)從35 ℃到900 ℃進行10次熱重循環后,LiF @ GO @ SiO2微膠囊[50]
Figure 5. SEM micrographs of microcapsules after heating: (a) LiF@PDA@SiO2 microcapsules without GO anti-corrosion leakage-proof layer being heated from 35 ℃ to 1000 ℃; (b) LiF@PDA@GO microcapsules without SiO2 heat-resistant strength layer being heated from 35 ℃ to 1000 ℃; (c) LiF@GO@SiO2 microcapsules being heated from 35 ℃ to 1000 ℃; (d) LiF@GO@SiO2 microcapsules after thermo-gravimetric circulation 10 times from 35 ℃ to 900 ℃[50]
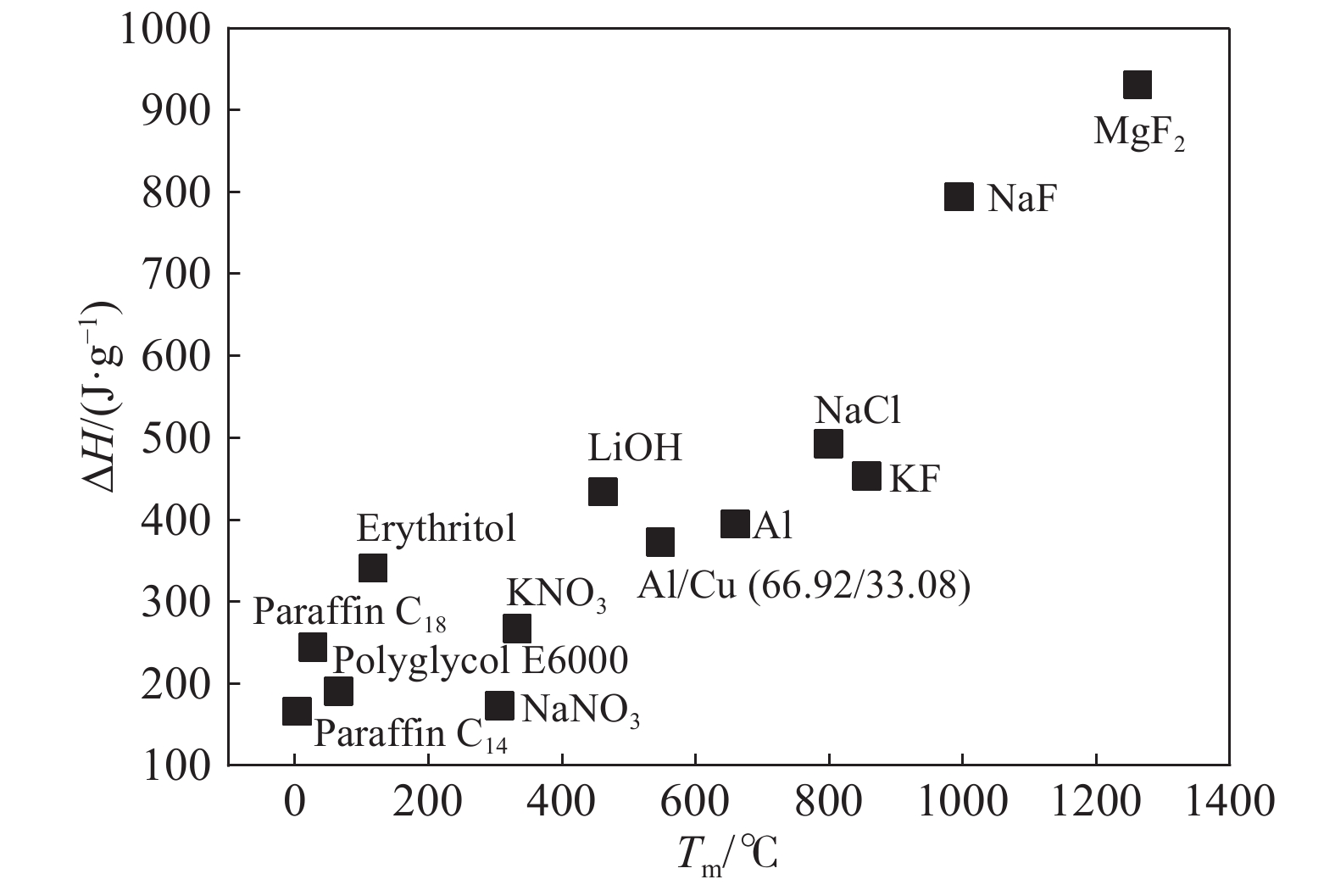
表 1 高溫相變材料的熔點和焓值
Table 1. Melting temperature and heat of fusion of high-temperature phase change materials
Material Melting temperature / ℃ Heat of fusion / (J·g–1) Material Melting temperature / ℃ Heat of fusion / (J·g–1) NaNO3 307 172 Mg 651 372.6 RbNO3 312 31 Al 660.1 393.6 Cd 320.9 54 FeCl2 677 337.9 NaOH 323 170 LiH 688 2678 KNO3 333 266 Li2MoO4 703 281 Zn/Mg(52/48) 340 180 MgCl2 714 454 KOH 380 149.7 Li2CO3 732 509 Zn/Al(96/4) 381 138 K 759 60.7 CsNO3 409 71 KCl 771 353 Zn 419 113 NaCl 800 492 AgBr 432 48.8 LiBO2 845 504.7 Mg/Cu/Zn(60/25/15) 452 254 LiF 848 1080 LiI 458 109 Cu/P/Si(83/10/7) 840 92 LiOH 462 433.4 Na2CO3 854 275.7 PbCl2 501 78.7 KF 857 452 Al/Cu/Mg/Zn(54/22/18/6) 520 305 ZnF2 872 400 SrI2 527 57 K2CO3 897 235.8 Al/Cu(66.92/33.08) 548 372 Si/Mg(56/44) 946 757 LiBr 550 203 NaF 996 794 Ca(NO3)2 560 145 NaMgF3 1022 670 Al/Cu/Si(65/30/5) 571 422 KCaF3 1070 460 Ba(NO3)2 594 209 KMgF3 1072 710 Sr(NO3)2 608 221 Cu 1083 205.2 LiCl 610 441 Na2SiO3 1088 424 CsI 629 96 MgF2 1263 930 MgI2 633 93 CaF2 1418 391 CsBr 638 105 CaSO4 1460 203 RbI 646 104 Fe 1535 314 SrBr2 650 41 SrSO4 1605 196 259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Dresselhaus M S, Thomas I L. Alternative energy technologies. Nature, 2001, 414(6861): 332 doi: 10.1038/35104599 [2] Zalba B, Marin J M, Cabeza L F, et al. Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl Therm Eng, 2003, 23(3): 251 doi: 10.1016/S1359-4311(02)00192-8 [3] Tuncbilek K, Sari A, Tarhan S, et al. Lauric and palmitic acids eutectic mixture as latent heat storage material for low temperature heating applications. Energy, 2005, 30(5): 677 doi: 10.1016/j.energy.2004.05.017 [4] Tan F L, Tso C P. Cooling of mobile electronic devices using phase change materials. Appl Therm Eng, 2004, 24(2-3): 159 doi: 10.1016/j.applthermaleng.2003.09.005 [5] Mondal S. Phase change materials for smart textiles–an overview. Appl Therm Eng, 2008, 28(11-12): 1536 doi: 10.1016/j.applthermaleng.2007.08.009 [6] Alva G, Lin Y X, Liu L K, et al. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: a review. Energy Build, 2017, 144: 276 doi: 10.1016/j.enbuild.2017.03.063 [7] Schossig P, Henning H M, Gschwander S, et al. Micro-encapsulated phase-change materials integrated into construction materials. Sol Energy Mater Sol Cells, 2005, 89(2-3): 297 doi: 10.1016/j.solmat.2005.01.017 [8] Zhang X X, Tao X M, Yick K L, et al. Structure and thermal stability of microencapsulated phase change materials. Colloid Polym Sci, 2004, 282(4): 330 doi: 10.1007/s00396-003-0925-y [9] Sari A, Alkan C, Karaipekli A, et al. Micro-encapsulated n-octacosane as phase change material for thermal energy storage. Sol Energy, 2009, 83(10): 1757 doi: 10.1016/j.solener.2009.05.008 [10] De Luca M, Ferraro M M, Hartmann R, et al. Advances in use of capsule-based fluorescent sensors for measuring acidification of endocytic compartments in cells with altered expression of V-ATPase subunitV1G1. ACS Appl Mater Interfaces, 2015, 7(27): 15052 doi: 10.1021/acsami.5b04375 [11] Guo P J, Weimer M S, Emery J D, et al. Conformal coating of a phase change material on ordered plasmonic nanorod arrays for broadband all-optical switching. ACS Nano, 2017, 11(1): 693 doi: 10.1021/acsnano.6b07042 [12] Ling Z Y, Zhang Z G, Shi G Q, et al. Review on thermal management systems using phase change materials for electronic components, Li-ion batteries and photovoltaic modules. Renew Sust Energ Rev, 2014, 31: 427 doi: 10.1016/j.rser.2013.12.017 [13] Xu B, Li P W, Chan C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: a review to recent developments. Appl Energ, 2015, 160: 286 doi: 10.1016/j.apenergy.2015.09.016 [14] Zhong Y J, Zhao B C, Lin J, et al. Encapsulation of high-temperature inorganic phase change materials using graphite as heat transfer enhancer. Renew Energ, 2019, 133: 240 doi: 10.1016/j.renene.2018.09.107 [15] Liu M, Bell S, Segarra M, et al. A eutectic salt high temperature phase change material: Thermal stability and corrosion of SS316 with respect to thermal cycling. Sol Energy Mater Sol Cells, 2017, 170: 1 doi: 10.1016/j.solmat.2017.05.047 [16] Jiang Y F, Sun Y P, Bruno F, et al. Thermal stability of Na2CO3?Li2CO3 as a high temperature phase change material for thermal energy storage. Thermochim Acta, 2017, 650: 88 doi: 10.1016/j.tca.2017.01.002 [17] Huang Z W, Xie N, Luo Z G, et al. Characterization of medium-temperature phase change materials for solar thermal energy storage using temperature history method. Sol Energy Mater Sol Cells, 2018, 179: 152 doi: 10.1016/j.solmat.2017.11.006 [18] Kenisarin M M. High-temperature phase change materials for thermal energy storage. Renew Sust Energ Rev, 2010, 14(3): 955 doi: 10.1016/j.rser.2009.11.011 [19] Hoshi A, Mills D R, Bittar A, et al. Screening of high melting point phase change materials (PCM) in solar thermal concentrating technology based on CLFR. Sol Energy, 2005, 79(3): 332 doi: 10.1016/j.solener.2004.04.023 [20] Nazir H, Batool M, Osorio F J B, et al. Recent developments in phase change materials for energy storage applications: a review. Int J Heat Mass Transfer, 2019, 129: 491 doi: 10.1016/j.ijheatmasstransfer.2018.09.126 [21] Nardin G, Meneghetti A, Dal Magro F, et al. PCM-based energy recovery from electric arc furnaces. Appl Energ, 2014, 136: 947 doi: 10.1016/j.apenergy.2014.07.052 [22] Fukahori R, Nomura T, Zhu C Y, et al. Thermal analysis of Al–Si alloys as high-temperature phase-change material and their corrosion properties with ceramic materials. Appl Energ, 2016, 163: 1 doi: 10.1016/j.apenergy.2015.10.164 [23] Sun J Q, Zhang R Y, Liu Z P, et al. Thermal reliability test of Al–34% Mg–6% Zn alloy as latent heat storage material and corrosion of metal with respect to thermal cycling. Energy Convers Manage, 2007, 48(2): 619 doi: 10.1016/j.enconman.2006.05.017 [24] Maruoka N, Akiyama T. Exergy recovery from steelmaking off-gas by latent heat storage for methanol production. Energy, 2006, 31(10-11): 1632 doi: 10.1016/j.energy.2005.05.023 [25] Jiang Y F, Sun Y P, Liu M, et al. Eutectic Na2CO3–NaCl salt: a new phase change material for high temperature thermal storage. Sol Energy Mater Sol Cells, 2016, 152: 155 doi: 10.1016/j.solmat.2016.04.002 [26] Jacob R, Liu M, Sun Y P, et al. Characterisation of promising phase change materials for high temperature thermal energy storage. J Energy Storage, 2019, 24: 100801 doi: 10.1016/j.est.2019.100801 [27] Misra A K, Whittenberger J D. Fluoride salts and container materials for thermal energy storage applications in the temperature range 973 to 1400 K // 22nd Intersociety Energy Conversion Engineering Conference. Philadelphia, Pennsylvania, 1987: 188 [28] Lund K O. Analysis of radiative and phase-change phenomena with application to space-based thermal energy storage. NASA Rep, 1991: 92 [29] Shi T J, Hu P, Wang J T. Preparation of polyurea microcapsules containing phase change materials using microfluidics. Chem Select, 2020, 5(7): 2342 [30] Feng L, Dong S P, Zhou H. n-Dodecanol nanocapsules with supramolecular lock shell layer for thermal energy storage. Chem Eng J, 2020, 389: 124483 doi: 10.1016/j.cej.2020.124483 [31] Xu R, Xia X M, Wang W, et al. Infrared camouflage fabric prepared by paraffin phase change microcapsule with Good thermal insulting properties. Colloids Surf A, 2020, 591: 124591 [32] Sahan N, Paksoy H. Designing behenic acid microcapsules as novel phase change material for thermal energy storage applications at medium temperature. Int J Energy Res, 2020, 44(5): 3922 doi: 10.1002/er.5193 [33] Wang X G, Chen Z F, Xu W, et al. Capric acid phase change microcapsules modified with graphene oxide for energy storage. J Mater Sci, 2019, 54(24): 14834 doi: 10.1007/s10853-019-03954-2 [34] Zhang H Z, Wang X D, Wu D Z. Silica encapsulation of n-octadecane via sol-gel process: a novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J Colloid Interf Sci, 2010, 343(1): 246 doi: 10.1016/j.jcis.2009.11.036 [35] Liu Z F, Chen Z H, Yu F. Preparation and characterization of microencapsulated phase change materials containing inorganic hydrated salt with silica shell for thermal energy storage. Sol Energy Mater Sol Cells, 2019, 200: art. No. 110004 doi: 10.1016/j.solmat.2019.110004 [36] He F, Wang X D, Wu D Z. New approach for sol-gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy, 2014, 67: 223 doi: 10.1016/j.energy.2013.11.088 [37] Li F N, Wang X D, Wu D Z. Fabrication of multifunctional microcapsules containing n-eicosane core and zinc oxide shell for low-temperature energy storage, photocatalysis, and antibiosis. Energy Convers Manage, 2015, 106: 873 doi: 10.1016/j.enconman.2015.10.026 [38] Sakai H, Sheng N, Kurniawan A, et al. Fabrication of heat storage pellets composed of microencapsulated phase change material for high-temperature applications. Appl Energ, 2020, 265: 114673 doi: 10.1016/j.apenergy.2020.114673 [39] Nomura T, Sheng N, Zhu C Y, et al. Microencapsulated phase change materials with high heat capacity and high cyclic durability for high-temperature thermal energy storage and transportation. Appl Energ, 2017, 188: 9 doi: 10.1016/j.apenergy.2016.11.025 [40] He F, Chao S, He X D, et al. Inorganic microencapsulated core/shell structure of Al?Si alloy micro-particles with silane coupling agent. Ceram Int, 2014, 40(5): 6865 doi: 10.1016/j.ceramint.2013.12.006 [41] Nomura T, Zhu C Y, Sheng N, et al. Microencapsulation of metal-based phase change material for high-temperature thermal energy storage. Sci Rep, 2015, 5: 9117 doi: 10.1038/srep09117 [42] Zhang M J, Han Z J, Gu H Z, et al. High Temperature Phase Change Thermal Storage Microcapsule of Dense Alumina Shell and Its Preparation Method: China Patent, CN201810202184.8. 2018-08-10張美杰, 韓藏娟, 顧華志, 等. 致密氧化鋁殼層高溫相變蓄熱微膠囊及其制備方法: 中國專利, CN201810202184.8. 2018-08-10 [43] Zhang F, Lin J, Yang X, et al. The Invention Relates to a Method for Preparing Metal Coated High-Temperature Phase Change Thermal Storage Microcapsule and the Thermal Storage Microcapsule Obtained Therefrom: China Patent, CN201811197058.4. 2019-02-12張鋒, 林俊, 楊旭, 等. 一種制備金屬包殼高溫相變儲熱微膠囊的方法以及由此得到的儲熱微膠囊: 中國專利, CN201811197058.4. 2019-02-12 [44] Yang Z Z, Zhang X, Wang Q. Metal and Alloy Phase Change Energy Storage Microcapsules and Their Preparation Methods: China Patent, CN201710129236.9. 2017-07-04楊振忠, 張行, 王倩. 金屬及合金相變儲能微膠囊及其制備方法: 中國專利, CN201710129236.9. 2017-07-04 [45] Li J F, Lu W, Luo Z P, et al. Thermal stability of sodium nitrate microcapsules for high-temperature thermal energy storage. Sol Energy Mater Sol Cells, 2017, 171: 106 doi: 10.1016/j.solmat.2017.06.028 [46] Takai-Yamashita C, Shinkai I, Fuji M, et al. Effect of water soluble polymers on formation of Na2SO4 contained SiO2 microcapsules by W/O emulsion for latent heat storage. Adv Powder Technol, 2016, 27(5): 2032 doi: 10.1016/j.apt.2016.07.012 [47] Li J F, Lu W, Luo Z P, et al. Synthesis and thermal properties of novel sodium nitrate microcapsules for high-temperature thermal energy storage. Sol Energy Mater Sol Cells, 2017, 159: 440 doi: 10.1016/j.solmat.2016.09.051 [48] Li J F, Luo Z P, Sun L L. The Invention Relates to an Inorganic Salt High-Temperature Phase Change Microcapsule and A Preparation Method Thereof: China Patent, CN201811091919.0. 2018-12-21李俊峰, 羅正平, 孫理理. 一種無機鹽高溫相變微膠囊及其制備方法: 中國專利, CN201811091919.0. 2018-12-21 [49] Li J F, Luo Z P, Wang M, et al. The Invention Relates to A Phase Change Thermal Control Coating and A Preparation Method Thereof: China Patent, CN201910569905.3. 2019-11-05李俊峰, 羅正平, 王萌, 等. 一種相變熱控涂層及其制備方法: 中國專利, CN201910569905.3. 2019-11-05 [50] Liu J, Li J F, Luo Z P, et al. A novel multiwalled LiF@GO@SiO2 microcapsule with high phase change temperature. Sol Energy Mater Sol Cells, 2019, 203: art. No. 110188 doi: 10.1016/j.solmat.2019.110188 [51] Zhang Q Y, Liu J, Liu Y B, et al. A Kind of High Temperature and High Enthalpy Phase Change Material Multi-Wall Microcapsule and Its Preparation Method: China Patent, CN201910483692.2. 2019-08-16張秋禹, 劉錦, 劉毅彬, 等. 一種高溫高焓值相變材料多壁結構微膠囊及制備方法: 中國專利, CN201910483692.2. 2019-08-16 [52] Zhang G C, Li J Q, Chen Y F, et al. Encapsulation of copper-based phase change materials for high temperature thermal energy storage. Sol Energy Mater Sol Cells, 2014, 128: 131 doi: 10.1016/j.solmat.2014.05.012 [53] He F, Song G P, He X D, et al. Structural and phase change characteristics of inorganic microencapsulated core/shell Al-Si/Al2O3 micro-particles during thermal cycling. Ceram Int, 2015, 41(9): 10689 doi: 10.1016/j.ceramint.2015.05.001 [54] Sheng N, Zhu C Y, Sakai H, et al. Synthesis of Al–25 wt% Si@Al2O3@Cu microcapsules as phase change materials for high temperature thermal energy storage. Sol Energy Mater Sol Cells, 2019, 191: 141 doi: 10.1016/j.solmat.2018.11.013 [55] Wang X F, Li C H, Zhao T. Fabrication and characterization of poly(melamine-formaldehyde)/silicon carbide hybrid microencapsulated phase change materials with enhanced thermal conductivity and light-heat performance. Sol Energy Mater Sol Cells, 2018, 183: 82 doi: 10.1016/j.solmat.2018.03.019 [56] Liao H H, Chen W H, Liu Y, et al. A phase change material encapsulated in a mechanically strong graphene aerogel with high thermal conductivity and excellent shape stability. Compos Sci Technol, 2020, 189: 108010 doi: 10.1016/j.compscitech.2020.108010 -




 下載:
下載: