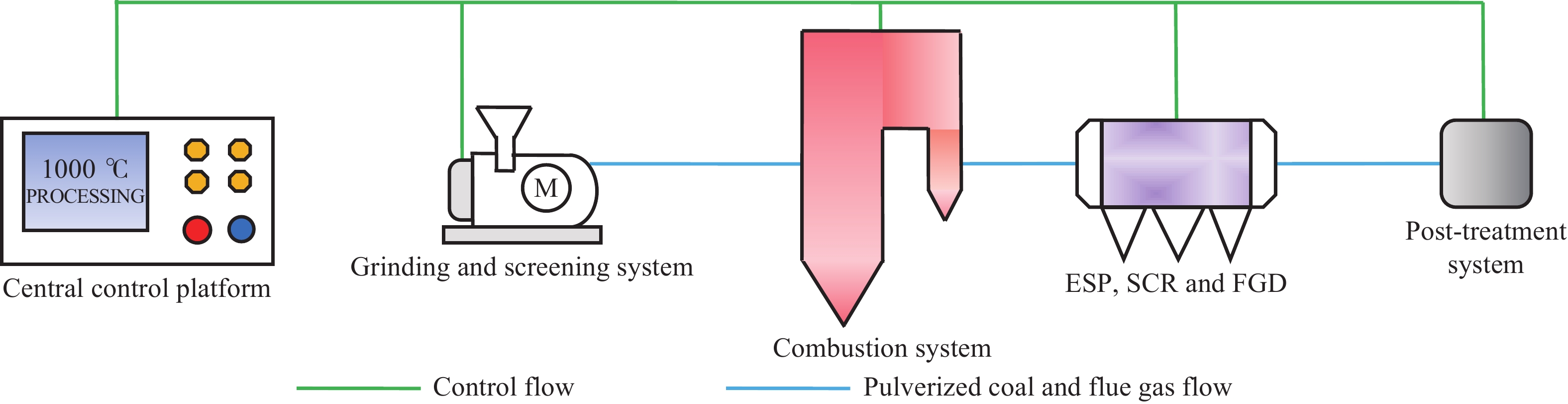
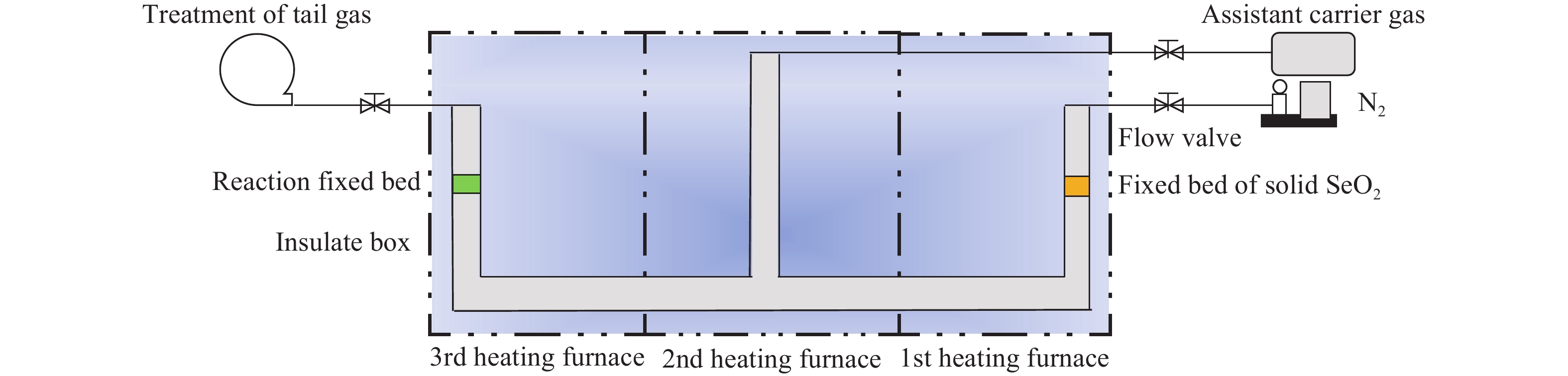
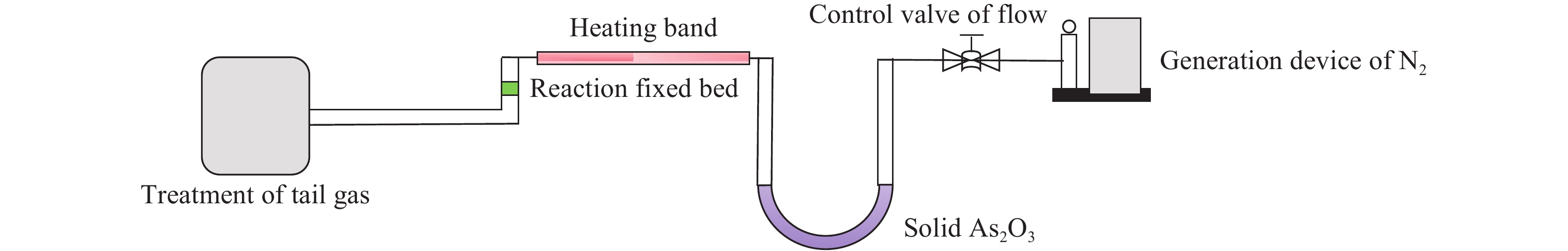
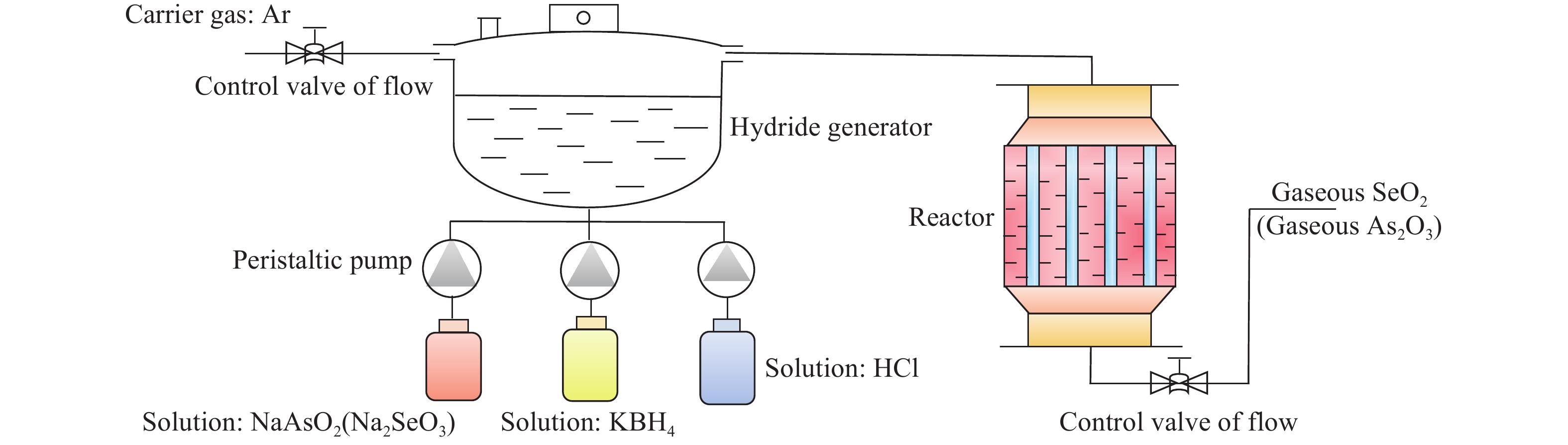
Research status of methods for generating gaseous trace element pollutants in simulated flue gas
-
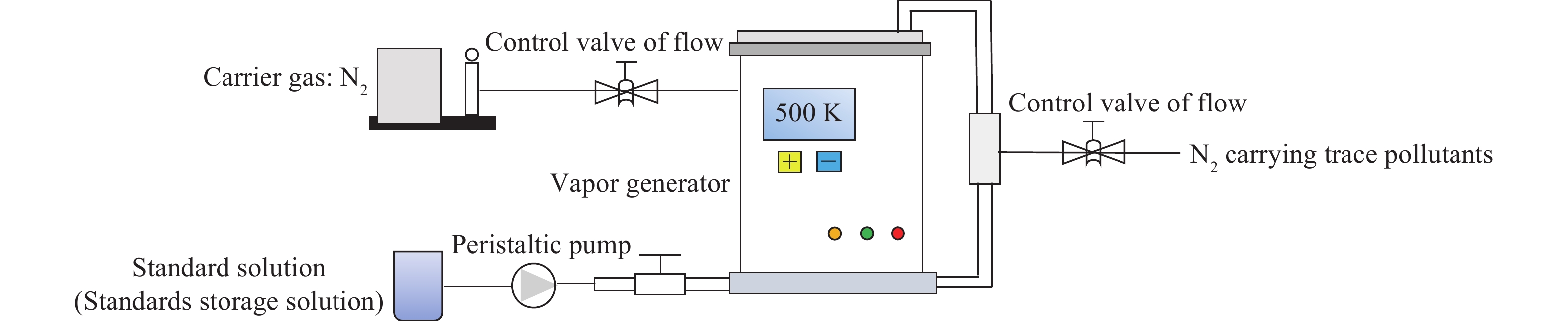
摘要: 目前燃煤電廠對于SO2、NOx和PM等主要污染物已經有較為成熟的控制方法,但針對具有長期環境危害性的痕量污染物尚缺乏有效的排放控制手段。為全面掌握痕量污染物在煤燃燒過程中的釋放、遷移和轉化規律并開發相應的控制技術,建立穩定可靠的模擬煙氣痕量污染物發生方法是開展相關研究的前提條件。通過文獻調研,對常見痕量污染物的四種發生方法進行了總結、歸納和對比:溶液蒸發法較為簡單易用,但產物中易含有副產物,這些副產物會帶來一定的影響;燃燒法產生的痕量污染物最接近實際情況,但受實驗條件影響較大,并且產物成分較為復雜;升華法獲得的產物濃度較為準確,但適用范圍較窄,僅用于某幾種氣態痕量污染物的發生;氫化物氧化法可準確地控制產物的發生速率,但也僅適用于少量痕量污染物,并且裝置較為復雜。分析比較了不同方法的適用情形,最后提出多種方法聯用的思路以期得到更加接近實際情形并且成分可控的結果。Abstract: Technologies for the emission and control of pollutants have been widely applied in coal-fired power plants in China to control the emissions of SO2, NOx, and particulate matter (PM). SO2 and NOx belong to the pollutants of major elements, with PM pertaining to the solid products. Control technologies for the above three pollutants, such as flue gas desulfurization, selective catalytic reduction, and electrostatic precipitators, have been proven to be highly efficient at removing the abovementioned pollutants in practical settings. The emission and control of Hg pollutants have also been extensively studied. However, control technologies for pollutants from trace elements, including Cd, Cr, Pb, Se, and As, which are also hazardous to long-term human health and the ecosystem, must be further developed both experimentally and theoretically. As a first step in future studies and analyses, the development of accurate and reliable methods for generating trace element pollutants is extremely important for the development of their future control technologies. In this paper, different ways of generating trace elements pollutants in simulated flue gas have been summarized and compared, including solution evaporation, combustion, sublimation, and hydride oxidization methods. The detailed procedures of these methods have been presented, and the advantages and disadvantages of each method have been discussed in detail. The findings indicate that the solution evaporation method is simple and feasible, but includes water vapor and other possible gaseous by-products that will have a negative effect on the results of subsequent experiments. The combustion method offers a realistic simulated flue gas, although factors related to the fuel or combustion conditions might influence the results and the product constituents are somewhat complex. The sublimation and hydride oxidation methods provide the most accurate trace element pollutants, but they are only suitable for the generation of certain types of gaseous trace pollutants and the installation of the hydride oxide apparatus is complicated. The applicability of these methods has also been discussed carefully in this study. A joint method for generating trace element pollutants has been proposed to obtain results that are closer to the actual situation and more precise than those obtained using any single method.
-
Key words:
- coal combustion /
- coal-fired flue gas /
- trace pollutants /
- evaporation /
- oxidation /
- generation methods
-
表 1 痕量元素化合物熱分解特性
Table 1. Thermolysis properties of trace element compounds
Elements Characteristics of thermolysis Cr[37?38] Cr(NO3)3 and Cr(CO)6 decompose to Cr2O3 and Cr at 373 K and 383 K, respectively[39]. Cr2O3 is stable below 2000 K[40]. CrCl3 and Cr2(SO4)3 are stable below 1200 K[40]. Pb[41?42] Pb(NO3)2 and Pb(CH3COO)2 decompose to Pb and PbO at 743 K and 473 K, respectively[39]. PbO is stable below 2000 K[40]. PbCl2 is stable below 1200 K[40]. Se[43] H2SeO4 decomposes to H2SeO3 at 533 K[44]. H2SeO3 decomposes to SeO2 at 343 K[45]. SeO2 is stable below 1500 K[40]. As[46?47] H3AsO4 decomposes to As2O5 at 433 K[39]. As2O5 decomposes to As2O3 at 588 K[39]. As2O3 is stable below 732 K[40]. Cd[48] CdCl2 is stable below 1236 K[40]. CdSO4 is stable below 1408 K[40]. 259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] China Electricity Council. List of basic data of power statistics from 2009 to 2018 [EB/OL]. China Electricity Council (2018-12-20) [2020-03-05]. https://cec.org.cn/menu/index.html?217中國電力企業聯合會. 2009-2018年電力統計基本數據一覽表[EB/OL]. 中國電力企業聯合會 (2018-12-20) [2020-03-05]. https://cec.org.cn/menu/index.html?217 [2] Li Z, Zhang Z Q, Lu M F, et al. Comparative analysis of coal consumption for thermal power generation. Sci Technol Innovation Herald, 2016(24): 42李澤, 張志強, 盧明飛, 等. 火電機組發電煤耗算例對比分析. 科技創新導報, 2016(24):42 [3] Zhang J Z, Chen Q Z, Ji J F, et al. Calculation method of pollutant emissions based on volume-to-heat ratio of coal-fired flue gas. Huadian Technol, 2017, 39(12): 50 doi: 10.3969/j.issn.1674-1951.2017.12.017張金柱, 陳啟召, 吉金芳, 等. 基于燃煤煙氣體積熱值比的污染物排放量計算方法. 華電技術, 2017, 39(12):50 doi: 10.3969/j.issn.1674-1951.2017.12.017 [4] General Office of the State Council of the People's Republic of China. Notice of the General Office of the State Council on Printing and Distributing the Pilot Work Plan for the Construction of “No Waste City” [EB/OL]. English.gov.cn (2019-01-21) [2010-03-05]. http://www.gov.cn/zhengce/content/2019-01/21/content_5359620.htm中華人民共和國國務院辦公廳. 國務院辦公廳關于印發“無廢城市”建設試點工作方案的通知 [EB/OL]. 中國政府網 (2019-01-21) [2020-03-05]. http://www.gov.cn/zhengce/content/2019-01/21/content_5359620.htm [5] Ministry of Environment Protection of the People’s Republic of China, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. GB 13223—2011 Emission Standard of Air Pollutants for Thermal Power Plants. Beijing: China Environmental Science Press, 2012中華人民共和國環境保護部, 中華人民共和國國家質量監督檢驗檢疫總局. GB 13223—2011 火電廠大氣污染物排放標準. 北京: 中國環境科學出版社, 2012 [6] The Central People’s Government of the People’s Republic of China. State Council Executive Meeting of the Central People’s Government of the People’s Republic of China [EB/OL]. English.gov.cn (2015-12-02) [2020-03-05]. http://www.gov.cn/guowuyuan/2015-12/02/content_5019050.htm中華人民共和國中央人民政府. 中華人民共和國中央人民政府國務院常務會議[EB/OL]. 中國政府網(2015-12-02) [2020-03-05]. http://www.gov.cn/guowuyuan/2015-12/02/content_5019050.htm [7] Sheng H C, Zhou W L, Lou J, et al. Practice of an ultra-low emission retrofit project for a coal-fired power plant. Environ Eng, 2019, 37(3): 124盛洪產, 周為莉, 樓軍, 等. 燃煤熱電廠煙氣超低排放改造工程實踐. 環境工程, 2019, 37(3):124 [8] Wu B H, Li S Y, Niu G P, et al. Synergistic removal technologies for flue gas pollutants and their engineering application in coal-fired units. Thermal power generation, 2017, 46(11): 103武寶會, 李帥英, 牛國平, 等. 燃煤機組煙氣污染物協同脫除技術及應用. 熱力發電, 2017, 46(11):103 [9] Zhao Y, Han L P. Synergistic removal of mercury by low-low temperature ESP for ultra-low emission coal-fired power plants. J Chin Soc Power Eng, 2019, 39(4): 319趙毅, 韓立鵬. 超低排放燃煤電廠低低溫電除塵器協同脫汞研究. 動力工程學報, 2019, 39(4):319 [10] Li J, Lü R T, Wang C. Experimental study on dry desulphurization of sodium bicarbonate. J Beijing Univ Technol, 2020, 46(4): 353李堅, 呂瑞彤, 王川. 碳酸氫鈉干法脫硫的實驗研究. 北京工業大學學報, 2020, 46(4):353 [11] Liang Q M, Liang W J, Fan X, et al. Effects of different factors on low-temperature NO conversion of V2O5?WO3?TiO2 catalysts. J Beijing Univ Technol, 2019, 45(7): 699梁全明, 梁文俊, 樊星, 等. 不同因素對V2O5?WO3?TiO2催化劑低溫NO轉化率的影響. 北京工業大學學報, 2019, 45(7):699 [12] Sun D M. Upgrading and Operating Performance of Ultra-Low Emission System for Coal-fired Power Plants Flue Gas [Dissertation]. Shijiazhuang: Hebei University of Science and Technology, 2018孫冬梅. 燃煤電廠煙氣超低排放升級改造及運行性能[學位論文]. 石家莊: 河北科技大學, 2018 [13] Chen J, Zheng Z M, Xu L, et al. Technical application analysis of ultra-low emission standard of flue gas. Appl Energy Technol, 2019(5): 39 doi: 10.3969/j.issn.1009-3230.2019.05.011陳堅, 鄭莊明, 徐林, 等. 實現煙氣超低排放標準的技術應用分析. 應用能源技術, 2019(5):39 doi: 10.3969/j.issn.1009-3230.2019.05.011 [14] Wang L. Study on the Partitioning Characteristics of Hg, As, Se and Other Hazardous Elements in Ultra-Low Emission Coal Fired Power Plants [Dissertation]. Hangzhou: Zhejiang University, 2018王麗. 超低排放機組中汞、砷和硒等重金屬的遷移特性研究[學位論文]. 杭州: 浙江大學, 2018 [15] Li Y, Zhang Y P. Study on the health risk assessment of pollutants from coal burning in Taiyuan. J Environ Occupational Med, 2005, 22(6): 506李巖, 張燕萍. 太原市燃煤污染物對人體健康的危險度評價. 環境與職業醫學, 2005, 22(6):506 [16] Kang Y. Environmental Biogeochemistry of Arsenic in Coal Mining Area [Dissertation]. Hefei: University of Science and Technology of China, 2014康彧. 煤礦區中砷的環境生物地球化學研究[學位論文]. 合肥: 中國科學技術大學, 2014 [17] Lin M. The Research on Environmental Impact Assessment in Coal-burning Power Plants [Dissertation]. Baoding: North China Electric Power University, 2008林明. 燃煤電廠環境影響評價研究[學位論文]. 保定: 華北電力大學, 2008 [18] The State Council of the People’s Republic of China. Implementation Regulations of the People’s Republic of China on Environmental Protection Tax Law [EB/OL]. English.gov.cn (2017-12-30) [2020-03-05]. http://www.gov.cn/zhengce/content/2017-12/30/content_5251797.htm中華人民共和國國務院. 中華人民共和國環境保護稅法實施條例 [EB/OL]. 中國政府網 (2017-12-30) [2020-03-05]. http://www.gov.cn/zhengce/content/2017-12/30/content_5251797.htm [19] United States Environmental Protection Agency. RIN 2060-AU70 Mercury and Air Toxics Standards for Power Plants Electronic Reporting Revisions. Research Triangle Park: United States Environmental Protection Agency, 2020 [20] Zhang P, Pan W G, Guo R T, et al. Advances in pollutants collaborative control technologies from coal-fired flue gas. Appl Chem Ind, 2017, 46(12): 2447 doi: 10.3969/j.issn.1671-3206.2017.12.039張萍, 潘衛國, 郭瑞堂, 等. 燃煤煙氣污染物協同控制技術的研究進展. 應用化工, 2017, 46(12):2447 doi: 10.3969/j.issn.1671-3206.2017.12.039 [21] Shu X, Shen Z Y, Sun Z Q, et al. Research and analysis of trace element migration and transformation in coal combustion // Seminar on Clean Combustion and Comprehensive Pollutants Treatment for Coal-fired Power Generation, China Power Engineering Society Environmental Protection Technology and Equipment Special Committee Annual Meeting in 2016. Shanghai, 2016: 251舒喜, 申智勇, 孫尊強, 等. 燃煤中痕量元素遷移轉化研究分析 // 2016年燃煤發電清潔燃燒與污染物綜合治理技術研討會、中國動力工程學會環保技術與裝備專委會年會論文集. 上海, 2016: 251 [22] Xu H, Zhang C, Yuan C L, et al. Study on arsenic adsorption characteristics by mineral elements in simulated flue gas atmosphere. J Fuel Chem Technol, 2019, 47(7): 876 doi: 10.3969/j.issn.0253-2409.2019.07.013許豪, 張成, 袁昌樂, 等. 模擬煙氣氣氛下礦物元素組分對砷的吸附特性研究. 燃料化學學報, 2019, 47(7):876 doi: 10.3969/j.issn.0253-2409.2019.07.013 [23] Zhang L. Research on Mercury Emission Measurement and Estimate from Combustion Resources [Dissertation]. Hangzhou: Zhejiang University, 2007張樂. 燃煤過程汞排放測試及汞排放量估算研究[學位論文]. 杭州: 浙江大學, 2007 [24] Yang H. Effects of calcium-based desulfurizing adsorbents on the emission of arsenic during coal combustion. J Wuhan Univ Technol, 2011, 33(3): 126 doi: 10.3963/j.issn.1671-4431.2011.03.027楊華. 鈣基固硫劑控制燃煤砷排放的實驗研究. 武漢理工大學學報, 2011, 33(3):126 doi: 10.3963/j.issn.1671-4431.2011.03.027 [25] Gu T X. Study on the Migration and Transformation of the Coal-Fired Pollutant in the Vadose Zone [Dissertation]. Changchun: Jilin University, 2018谷天雪. 燃煤電廠污染物組分在包氣帶中遷移轉化規律研究[學位論文]. 長春: 吉林大學, 2018 [26] Guo S L. Research on the Characteristics of Movement and Transformation and Pollution Control of Heavy Metals in Coal Combustion [Dissertation]. Chongqing: Chongqing University, 2014郭勝利. 燃煤重金屬遷移轉化特征及其污染控制研究[學位論文]. 重慶: 重慶大學, 2014 [27] He S. Experimental and Mechanism Study on Mercury Catalytic Oxidation in Coal-fired Flue Gas [Dissertation]. Hangzhou: Zhejiang University, 2009何勝. 燃煤煙氣汞催化氧化的試驗和機理研究[學位論文]. 杭州: 浙江大學, 2009 [28] Li S, Rao Z. Uncertainty evaluation for the results from the preparation of cadmium standard solution. Rock Miner Anal, 2009, 28(5): 479 doi: 10.3969/j.issn.0254-5357.2009.05.016李松, 饒竹. 鎘標準溶液配制結果的不確定度評定. 巖礦測試, 2009, 28(5):479 doi: 10.3969/j.issn.0254-5357.2009.05.016 [29] Xu C F. Study on Detection Methods and Pretreatment of Lead, Cadmium, Mercury and Arsenic in Corn [Dissertation]. Harbin: Heilongjiang University, 2014徐春峰. 玉米中重金屬鉛鎘汞砷檢測方法及前處理研究[學位論文]. 哈爾濱: 黑龍江大學, 2014 [30] State Administration for Market Regulation, National Institute of Metrology, China. National Sharing Platform for Reference Materials [EB/OL]. National Institute of Metrology, China (2020-01-01) [2020-03-05]. https://www.ncrm.org.cn/Web/Home/Index國家市場監督管理總局, 中國計量科學研究院. 國家標準物質資源共享平臺[EB/OL]. 中國計量科學研究院 (2020-01-01)[2020-03-05]. https://www.ncrm.org.cn/Web/Home/Index [31] Lei T. Nickel Sulfate Solution MVR Evaporator Control System Design [Dissertation]. Lanzhou: Lanzhou University of Technology, 2018雷霆. 硫酸鎳溶液MVR蒸發器控制系統研究與應用[學位論文]. 蘭州: 蘭州理工大學, 2018 [32] Xia B, He F, Zhang L, et al. Summary of ferric chloride acid solution evaporation system. China Chlor-Alkali, 2019(2): 21 doi: 10.3969/j.issn.1009-1785.2019.02.008夏斌, 何飛, 張龍, 等. 三氯化鐵酸性溶液蒸發系統概述. 中國氯堿, 2019(2):21 doi: 10.3969/j.issn.1009-1785.2019.02.008 [33] Li Z P, Ding R, Zhou H W, et al. Study on the growth of Levolucosan single crystal by solution evaporation method. J Synth Crystals, 2018, 47(11): 2300 doi: 10.3969/j.issn.1000-985X.2018.11.012李志鵬, 丁瑞, 周恒為, 等. 溶液蒸發法左旋葡聚糖單晶生長的研究. 人工晶體學報, 2018, 47(11):2300 doi: 10.3969/j.issn.1000-985X.2018.11.012 [34] Han X W, Zou T H, Chen J B, et al. Experimental study on evaporation of LiCl solution in low vacuum. Chin J Vac Sci Technol, 2019, 39(9): 775韓曉婉, 鄒同華, 陳劍波, 等. LiCl溶液真空蒸發過程的實驗研究. 真空科學與技術學報, 2019, 39(9):775 [35] Chen X B, Yu Y J, Ye J Y. Research on accurate control method of steam quantity of steam generator. Mod Manuf Technol Equip, 2018(7): 56 doi: 10.3969/j.issn.1673-5587.2018.07.024陳旭波, 余云加, 葉江勇. 蒸汽發生器蒸汽量的精確控制方法研究. 現代制造技術與裝備, 2018(7):56 doi: 10.3969/j.issn.1673-5587.2018.07.024 [36] Su F, Yang Q, Yue X F, et al. Research on steam generator based on heat pump. Energy Conservation, 2017, 36(2): 62蘇帆, 楊強, 岳獻芳, 等. 基于熱泵的蒸汽發生裝置研究. 節能, 2017, 36(2):62 [37] National Institute of Standards and Technology. NIST Chemistry WebBook [DB/OL]. National Institute of Standards and Technology (2018-12-31) [2020-03-05]. http://webbook.nist.gov/chemistry/ [38] National Center for Biotechnology Information. PubChem Compound [DB/OL]. National Center for Biotechnology Information (2020-01-31) [2020-03-05]. https://www.ncbi.nlm.nih.gov/pccompound [39] ChemNet of China. Chem YQ [DB/OL]. ChemNet of China (2020-01-31) [2020-03-05]. http://www.chemyq.com/xz.htm中國化工網. 化工引擎 [DB/OL]. 中國化工網 (2020-01-31) [2020-03-05]. http://www.chemyq.com/xz.htm [40] Ihsan B. Thermochemical Data of Pure Substances. Beijing: Science Press, 2003伊赫桑·巴倫. 純物質熱化學數據手冊. 北京: 科學出版社, 2003 [41] Beijing Chemical Book NetTech Ltd. Chemical Book [DB/OL]. Beijing Chemical Book NetTech Ltd (2020-01-20) [2020-03-05]. https://www.chemicalbook.com/ProductIndex.aspx北京西林布克網絡科技有限公司. 化學手冊 [DB/OL]. 北京西林布克網絡科技有限公司 (2020-01-20) [2020-03-05]. https://www.chemicalbook.com/ProductIndex.aspx [42] DrugFuture. Chemical Index Database [DB/OL]. DrugFuture (2020-01-20) [2020-03-05]. https://www.drugfuture.com/chemdata/藥物在線平臺. 化學物質索引數據庫 [DB/OL]. 藥物在線平臺 (2020-01-20) [2020-03-05] https://www.drugfuture.com/chemdata/ [43] Mark W. The periodic table of the elements [DB/OL]. WebElements (2020-01-30) [2020-03-05]. https://www.webelements.com [44] Shanghai Wingch chemical engineering technology Ltd. Basechem [DB/OL]. Shanghai Wingch chemical engineering technology Ltd (2020-01-31) [2020-03-05]. http://www.basechem.org/上海物競化工科技有限公司. 物競數據庫 [DB/OL]. 上海物競化工科技有限公司 (2020-01-31) [2020-03-05]. http://www.basechem.org/ [45] Beijing Zhitu Technology Ltd. SOMSDS [DB/OL]. Beijing Zhitu Technology Ltd (2020-01-30) [2020-03-05]. http://www.somsds.com/北京之途科技有限公司. SOMSDS [DB/OL]. 北京之途科技有限公司 (2020-01-30) [2020-03-05]. http://www.somsds.com/ [46] Zhang Y, Wang C B, Li W H, et al. Removal of gas-phase As2O3 by metal oxide adsorbents: effects of experimental conditions and evaluation of adsorption mechanism. Energy Fuels, 2015, 29(10): 6578 doi: 10.1021/acs.energyfuels.5b00948 [47] Du X S, Tang J Y, Chen Y R, et al. Adsorption of As4O6 from flue gas by zeolites: Influence of pore structure and Al substitution. Microporous Mesoporous Mater, 2017, 243: 22 doi: 10.1016/j.micromeso.2017.02.026 [48] Speight J G. Lange's Handbook of Chemistry. 16th Ed. New York: McGraw-Hill Companies, 2004 [49] National Institute of Metrology, China. Certificate of reference materials (arsenic acid solution) [EB/OL]. National Institute of Metrology, China (2018-04) [2020-03-05]. https://www.ncrm.org.cn/Repository/2732d687-5ae0-47db-8a8a-6d5c85a2f3c2.pdf中國計量科學研究院. 標準物質證書(砷酸根溶液標準物質) [EB/OL]. 中國計量科學研究院 (2018-04) [2020-03-05]. https://www.ncrm.org.cn/Repository/2732d687-5ae0-47db-8a8a-6d5c85a2f3c2.pdf [50] Li X Y, Li Y, Jin L J, et al. Removal of Hg0 from simulated flue gas by MnOx modified activated carbon. CIESC J, 2019, 70(8): 3078李向陽, 李揚, 靳立軍, 等. MnOx改性活性炭用于模擬煙氣中Hg0的脫除. 化工學報, 2019, 70(8):3078 [51] Chen C M, Nie G X, Jia W B, et al. Catalytic oxidation of elemental mercury over CuO?WO3/TiO2 in simulated coal-fired flue gas. Electr Power, 2019, 52(6): 179陳傳敏, 聶國欣, 賈文波, 等. CuO?WO3/TiO2催化氧化燃煤煙氣中Hg0試驗研究. 中國電力, 2019, 52(6):179 [52] Zhang X F, Yao Q, Song Q, et al. Experimental study on the emission characteristics of lead during combustion. Proc CSEE, 2007, 27(32): 18 doi: 10.3321/j.issn:0258-8013.2007.32.004張小鋒, 姚強, 宋薔, 等. 燃燒中鉛元素排放特性的實驗研究. 中國電機工程學報, 2007, 27(32):18 doi: 10.3321/j.issn:0258-8013.2007.32.004 [53] Zhou C C, Liu G J, Xu Z Y, et al. Effect of ash composition on the partitioning of arsenic during fluidized bed combustion. Fuel, 2017, 204: 91 doi: 10.1016/j.fuel.2017.05.048 [54] Liu H B. Size Distribution of Heavy Metals in Particles Emitted from Domestic Coal Burning and the Establishment of Emission Inventory [Dissertation]. Nanjing: Nanjing University of Information Science and Technology, 2017劉海彪. 民用燃煤排放顆粒物中重金屬粒徑分布及清單構建[學位論文]. 南京: 南京信息工程大學, 2017 [55] Huang Q. Effect of Mineral on the Particulate Formation and Ash Deposition during Pulverized Coal Combustion [Dissertation]. Beijing: Tsinghua University, 2017黃騫. 礦物質對煤粉燃燒顆粒物生成和沉積特性的影響機理[學位論文]. 北京: 清華大學, 2017 [56] Cheng Y L, Shi H B, Zhang N, et al. Comparative experiments of two high sodium coal mixed burning slagging characteristics. J China Coal Soc, 2017, 42(11): 3021程友良, 施宏波, 張寧, 等. 兩種高鈉煤的摻燒結渣特性對比實驗. 煤炭學報, 2017, 42(11):3021 [57] Liu W, Lü T, Tao M J. ICP-MS determination of 19 trace elements in graphene powder materials with microwave digestion. Phys Test Chem Anal (Part B Chem Anal) , 2019, 55(11): 1318劉巍, 呂婷, 陶美娟. 微波消解?電感耦合等離子體質譜法測定石墨烯粉末材料中19種痕量元素. 理化檢驗(化學分冊), 2019, 55(11):1318 [58] Li J B. Study on Head Transfer and Dynamic Characteristics of Heat Recovery Steam Generator in Multi-Component Complex Medium Condition [Dissertation]. Jinan: Shandong University, 2017李金波. 多組分復雜介質余熱鍋爐傳熱與動態特性研究[學位論文]. 濟南: 山東大學, 2017 [59] Lou Y. Experimental Study on the Promotion by Steam on CaO Adsorption SeO2 at Medium Temperature Range [Dissertation]. Beijing: Tsinghua University, 2018樓宇. 中低溫水蒸氣促進氧化鈣固硒實驗及機理研究[學位論文]. 北京: 清華大學, 2018 [60] Sterling R O, Helble J J. Reaction of arsenic vapor species with fly ash compounds: kinetics and speciation of the reaction with calcium silicates. Chemosphere, 2003, 51(10): 1111 doi: 10.1016/S0045-6535(02)00722-1 [61] Li Y Z, Tong H L, Zhuo Y Q, et al. Simultaneous removal of SO2 and trace SeO2 from flue gas: Effect of product layer on mass transfer. Environ Sci Technol, 2006, 40(13): 4306 doi: 10.1021/es052381s [62] Li Y Z. Experimental Study on Simultaneous Removal of Trace Selenium and Arsenic in Flue Gas Desulphurization within Medium Temperature Range [Dissertation]. Beijing: Tsinghua University, 2006李玉忠. 中溫脫硫過程聯合脫除痕量硒、砷的實驗研究[學位論文]. 北京: 清華大學, 2006 [63] Agnihotri R, Chauk S, Mahuli S, et al. Selenium removal using Ca-based sorbents: reaction kinetics. Environ Sci Technol, 1998, 32(12): 1841 doi: 10.1021/es971119j [64] Zhang H H, Wang Y Z, Tao W Q. Investigation of forced convective heat transfer using naphthalene sublimation technique. J Eng Thermophys, 1985, 6(1): 49張惠華, 王允中, 陶文銓. 強制對流換熱的萘昇華模擬研究. 工程熱物理學報, 1985, 6(1):49 [65] Chen M, Xie J C, Ji Y, et al. Applicability research of convective heat transfer coefficients mjeasured with the naphthalene sublimation method under conditions of high temperature and strong radiation. Build Sci, 2019, 35(12): 62陳默, 謝靜超, 姬穎, 等. 高溫、強輻射條件下萘升華法測量對流換熱系數的適用性研究. 建筑科學, 2019, 35(12):62 [66] Jia W H. Numerical Analysis on the Heat Transfer and Flow Characteristics of Condensation in Special Type Heat Exchange Tube about the Mixed Gas [Dissertation]. Jinan: Shandong University, 2019賈文華. 異形管內混合氣體流動凝結換熱特性數值模擬[學位論文]. 濟南: 山東大學, 2019 [67] Liu Z Y, Lu W, Jin X, et al. Study of dry-out characteristics of carbon dioxide during flow boiling in tube. CIESC J, 2019, 70(12): 4575劉忠彥, 逯瑋, 金旭, 等. CO2管內流動沸騰干涸特性研究. 化工學報, 2019, 70(12):4575 [68] Kong M. Study on Synergetic Deactivation Mechanism of Mercury, Arsenic and Potassium in Coal-Fired Flue Gas on V2O5?WO3/TiO2 Catalyst [Dissertation]. Chongqing: Chongqing University, 2018.孔明. 燃煤煙氣中汞砷與鉀對V2O5?WO3/TiO2脫硝催化劑協同作用失活機制研究[學位論文]. 重慶: 重慶大學, 2018 [69] Yu X H, Zhang Y, Chang L, et al. Se partitioning and species in WFGD and WESP system of the ultra-low emission coal-fired power plant. Clean Coal Technol, 2019, 25(3): 100余學海, 張翼, 常林, 等. 超低排放燃煤電站濕法脫硫和濕式電除塵器中硒含量分布及形態演變. 潔凈煤技術, 2019, 25(3):100 [70] Beijing Shuguangming Electronics Instrument Co., Ltd. Hydride generator-type A [EB/OL]. Beijing Shuguangming Electronics Instrument Co., Ltd (2020-01-30) [2020-03-05]. http://www.shuguangming.com/cn/pd.jsp?id=22#_pp=103_330北京曙光明電子光源儀器有限公司. 氫化物發生器A型 [EB/OL]. 北京曙光明電子光源儀器有限公司 (2020-01-30) [2020-03-05]. http://www.shuguangming.com/cn/pd.jsp?id=22#_pp=103_330 [71] Chen D K, Hu H Y, Xu Z, et al. Findings of proper temperatures for arsenic capture by CaO in the simulated flue gas with and without SO2. Chem Eng J, 2015, 267: 201 doi: 10.1016/j.cej.2015.01.035 [72] Hu H Y, Chen D K, Liu H, et al. Adsorption and reaction mechanism of arsenic vapors over gamma-Al2O3 in the simulated flue gas containing acid gases. Chemosphere, 2017, 180: 186 doi: 10.1016/j.chemosphere.2017.03.114 [73] Huang Y D, Yang Y H, Hu H Y, et al. A deep insight into arsenic adsorption over γ-Al2O3 in the presence of SO2/NO. Proc Combust Inst, 2019, 37(3): 2951 doi: 10.1016/j.proci.2018.06.136 -




 下載:
下載: