Review on the application of MOF materials for removal of pollutants from the water (II)
-
摘要: 對近年來MOF材料去除水環境中重金屬、有機物的相關研究進行了總結與評述。本篇是該主題的第2篇,主要對MOF材料去除水中有機污染物的相關研究進行總結和論述。研究表明,MOF材料含有大量開放性金屬位點、路易斯酸堿位以及官能團,因而對染料、抗生素、農藥、持久性有機污染物等均具有較高的吸附性能。氫鍵、π?π作用、疏水作用和靜電引力是其吸附有機污染物的主要機制,部分MOF材料中較大的孔道結構也有利于大分子有機污染物的吸附;另外,部分MOF材料還具有優異的催化性能,能夠作為類Fenton催化,光催化以及過硫酸鹽活化的催化劑實現對有機污染物的催化降解,其中光催化反應中污染物的降解主要源于·O2?、·OH和h+的貢獻;而在過硫酸鹽體系中,·O2?、·OH、SO4·?和1O2是導致有機污染物分解的主要活性氧化物種。基于對先前研究的回顧,相信未來的研究領域包括但不限于以下方面:(1)進一步提高MOF在去除有機污染物方面的性能,并提高其可回收性;(2)開展新型MOF催化材料的制備及催化反應機理的研究;(3)研究MOF缺陷結構的調控,以開發具有更高吸附和催化性能的新型MOF材料;(4)研究新的框架材料,例如共價有機骨架(COFs)材料,并將其應用于污染物凈化領域。Abstract: Metal–organic frameworks (MOFs) are a class of organic–inorganic hybrid functional materials generally formed via self-assembly of metal ions or metal clusters and rigid organic ligands with nitrogen and oxygen atoms. The wide range of potential applications of MOF materials includes gas storage and separation, catalysis, sensing, and drug transportation and release, which can be attributed to their versatile designable structures, modifiable chemical functionality, low-density framework, large specific surface area, and functional and permanent pore space. MOF and its composite materials have also been employed to remove various contaminants from the environment in the recent decade. To present the remarkable research progress and outcomes of MOF materials in the removal of pollutants from the water environment, the related studies on the removal of heavy metals and organic pollutants from the water environment were reviewed in this paper. This was the second paper on the topic that mainly introduced the research progress of MOF materials in the removal of organic pollutants in aqueous solution. The previous studies have shown that MOF materials have open metal sites and Lewis acid–base sites; thus, they exhibit a high adsorption performance for dyes, antibiotics, pesticides, and persistent organic pollutants. Hydrogen bonding, π–π interaction, hydrophobic interaction, and electrostatic attraction are the main mechanisms for their adsorption of organic pollutants. In addition, the large pore structure of some MOF materials is conducive to the adsorption of macromolecular organic pollutants. Moreover, some MOF materials that can be used as catalysts for Fenton-like reactions, photocatalytic reactions, and persulfate activation to degrade organic pollutants, exhibit excellent catalytic performance. The degradation of pollutants in photocatalytic reactions can be mainly attributed to the contributions of ·O2?, ·OH, and h+. In the persulfate system, ·O2?, ·OH, SO4·?, and 1O2 are the main reactive oxide species that cause the decomposition of organic pollutants. Based on the review of previous studies, it is believed that future research will include but will not be limited to the following: (1) the improvement of the performance of MOF in removing organic pollutants and its recyclability; (2) the preparation of new MOF catalytic materials and investigation of catalytic reaction mechanisms; (3) the regulation of the defect structure of MOF to develop new MOF materials with high adsorption and catalytic efficiency; and (4) the analysis of new framework materials, e.g., covalent organic framework materials, and their applications in the field of pollutant purification.
-
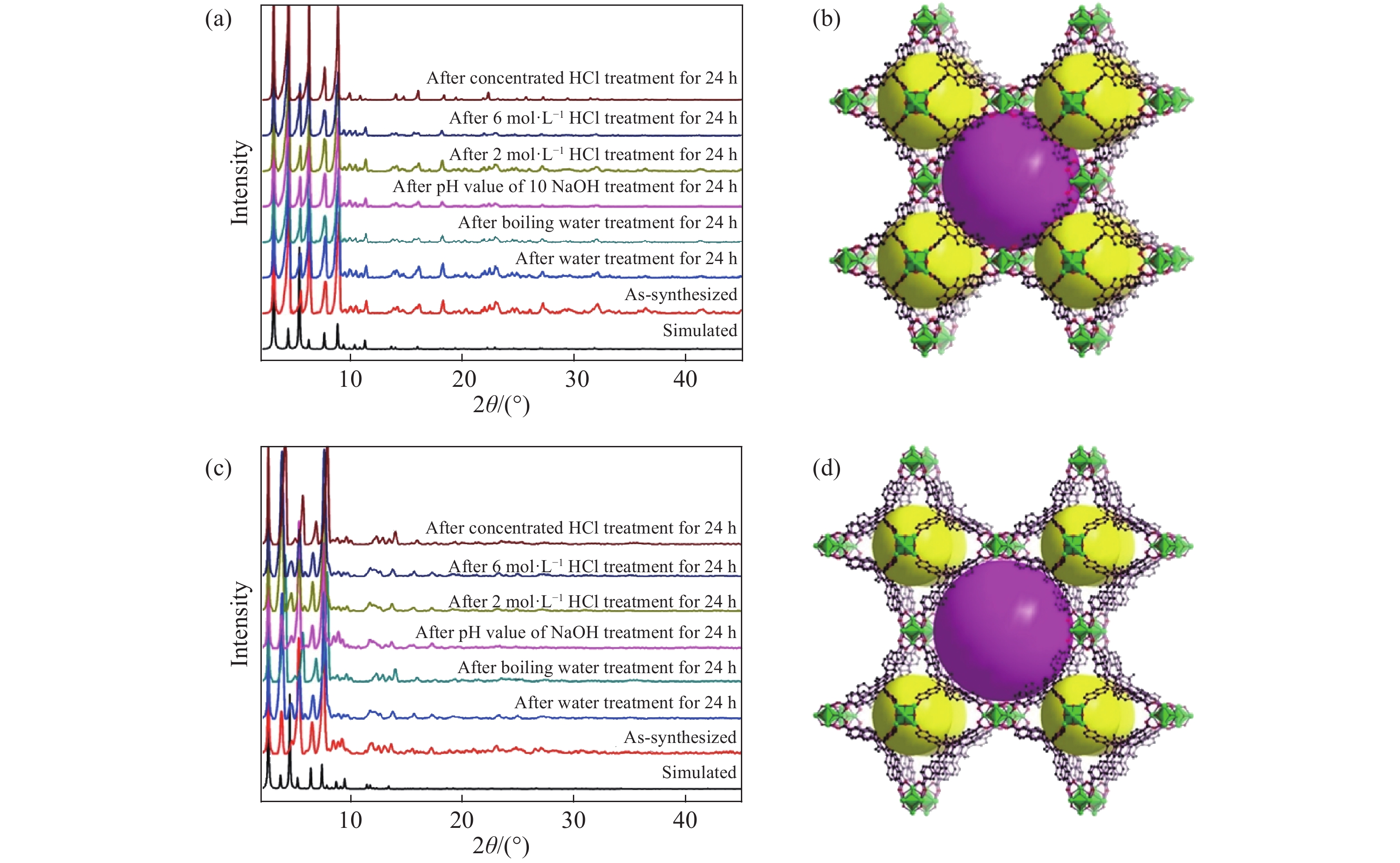
圖 2 BUT結構以及不同BUT樣品的粉末X射線衍射圖譜。(a)合成的BUT-12以及采取不同處理手段后得到的樣品的粉末X射線衍射圖譜;(b)BUT-12結構;(c)BUT-13以及采取不同處理手段后得到的樣品的粉末X射線衍射圖譜;(d)BUT-13結構[36]
Figure 2. BUT structure and powder X-ray diffraction (PXRD) patterns of different BUT samples: (a) PXRD patterns of BUT-12 upon treatment using different methods; (b) structures of BUT-12; (c) PXRD patterns of BUT-13 upon treatment using different methods; (d) structures of BUT-13[36]
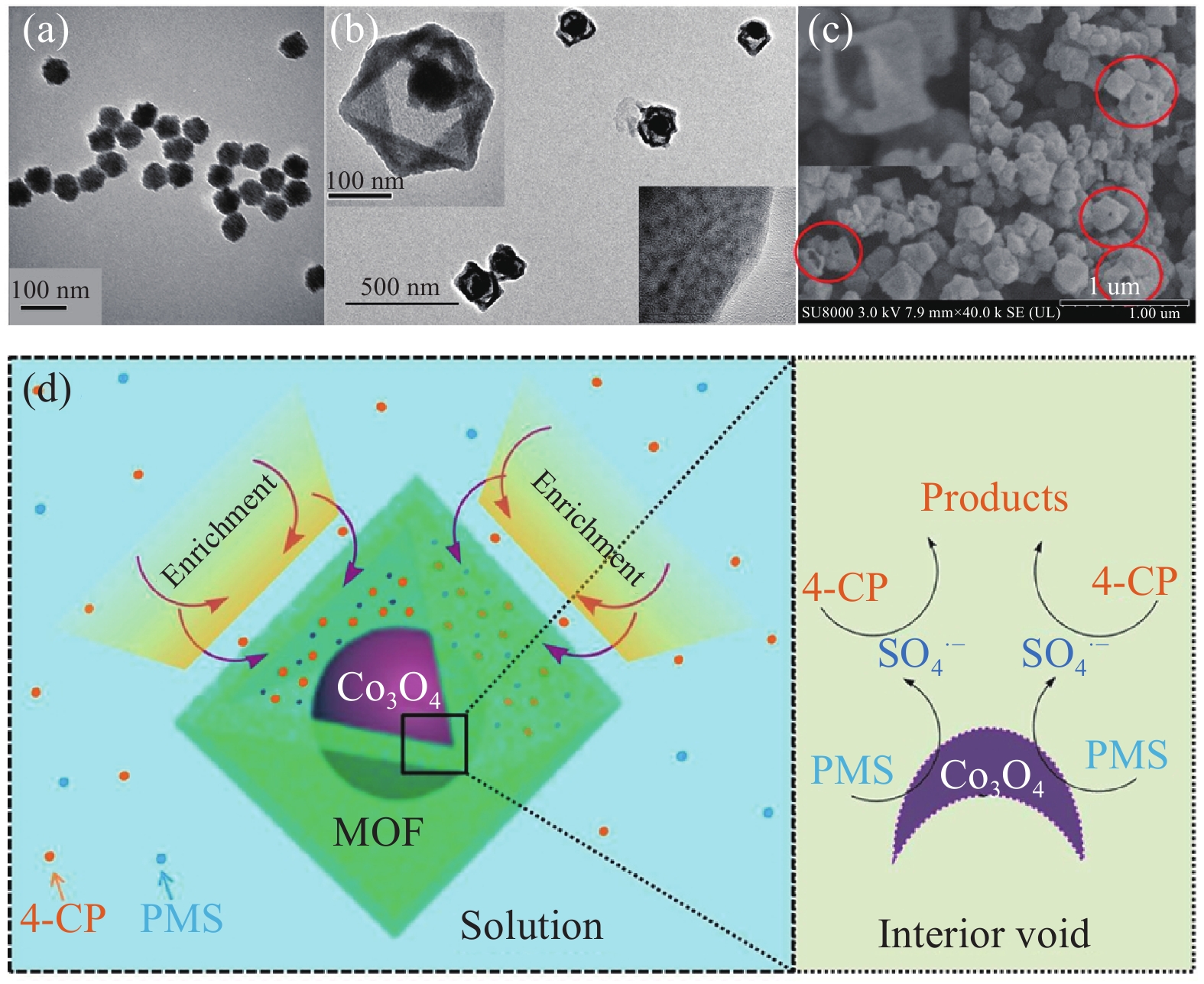
圖 7 不同催化劑的電鏡照片及污染物降解機理。Co3O4(a)和Co3O4@MOF-5(b)的透射電鏡圖像;(c)Co3O4@MOF-5的掃描電鏡圖像;(d)可能的降解機理[82]
Figure 7. Electron micrographs of different catalysts and degradation mechanism of pollutants: TEM images of Co3O4 (a) and Co3O4@MOFs (b); (c) SEM image of Co3O4@MOF-5; (d) possible mechanism of degradation[82]
259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Yaghi O M, Li G, Li H. Selective binding and removal of guests in a microporous metal-organic framework. Nature, 1995, 378(6558): 703 doi: 10.1038/378703a0 [2] Kitagawa S, Kitaura R, Noro S I. Functional porous coordination polymers. Angew Chem Int Ed, 2004, 43(18): 2334 doi: 10.1002/anie.200300610 [3] Janiak C. Functional organic analogues of zeolites based on metal-organic coordination frameworks. Angew Chem Int Ed, 1997, 36(13-14): 1431 [4] Li H L, Eddaoudi M, O'Keeffe M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature, 1999, 402(6759): 276 doi: 10.1038/46248 [5] Park K S, Ni Z, C?té A P, et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc Natl Acad Sci, 2006, 103(27): 10186 doi: 10.1073/pnas.0602439103 [6] Chen X, Jiang H, Hou B, et al. Boosting chemical stability, catalytic activity, and enantioselectivity of metal–organic frameworks for batch and flow reactions. J Am Chem Soc, 2017, 139(38): 13476 doi: 10.1021/jacs.7b06459 [7] Zhou X P, Li M, Liu J, et al. Gyroidal metal–organic frameworks. J Am Chem Soc, 2012, 134(1): 67 doi: 10.1021/ja208469n [8] He H M, Sun Q, Gao W Y, et al. A stable metal–organic framework featuring a local buffer environment for carbon dioxide fixation. Angew Chem Int Ed, 2018, 57(17): 4657 doi: 10.1002/anie.201801122 [9] Rajak R, Saraf M, Mohammad A, et al. Design and construction of a ferrocene based inclined polycatenated Co-MOF for supercapacitor and dye adsorption applications. J Mater Chem A, 2017, 5(34): 17998 doi: 10.1039/C7TA03773B [10] Zou X Y, Chen M, Cao X Q, et al. Review of application of MOF materials for removal of environmental pollutants from water (I). Chin J Eng, 2020, 42(3): 289鄒星云, 陳明, 曹曉強, 等. MOF材料在水環境污染物去除方面的應用現狀及發展趨勢(I). 工程科學學報, 2020, 42(3):289 [11] Haque E, Lee J E, Jang I T, et al. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J Hazard Mater, 2010, 181(1-3): 535 doi: 10.1016/j.jhazmat.2010.05.047 [12] Haque E, Jun J W, Jhung S H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J Hazard Mater, 2011, 185(1): 507 doi: 10.1016/j.jhazmat.2010.09.035 [13] Lin S, Song Z L, Che G B, et al. Adsorption behavior of metal-organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater, 2014, 193: 27 doi: 10.1016/j.micromeso.2014.03.004 [14] Lin K Y A, Chang H A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere, 2015, 139: 624 doi: 10.1016/j.chemosphere.2015.01.041 [15] Huo S H, Yan X P. Metal-organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J Mater Chem, 2012, 22(15): 7449 doi: 10.1039/c2jm16513a [16] Wang K K, Li C F, Liang Y X, et al. Rational construction of defects in a metal-organic framework for highly efficient adsorption and separation of dyes. Chem Eng J, 2016, 289: 486 doi: 10.1016/j.cej.2016.01.019 [17] Yue Y F, Qiao Z A, Fulvio P F, et al. Template-free synthesis of hierarchical porous metal-organic frameworks. J Am Chem Soc, 2013, 135(26): 9572 doi: 10.1021/ja402694f [18] Huang H L, Li J R, Wang K K, et al. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nature Commun, 2015, 6: 8847 doi: 10.1038/ncomms9847 [19] Tanaka S, Miyashita R. Aqueous-system-enabled spray-drying technique for the synthesis of hollow polycrystalline ZIF-8 MOF particles. ACS Omega, 2017, 2(10): 6437 doi: 10.1021/acsomega.7b01325 [20] Dadfarnia S, Shabani A M H, Moradi S E, et al. Methyl red removal from water by iron based metal-organic frameworks loaded onto iron oxide nanoparticle adsorbent. Appl Surf Sci, 2015, 330: 85 doi: 10.1016/j.apsusc.2014.12.196 [21] Bibi R, Wei L F, Shen Q H, et al. Effect of amino functionality on the uptake of cationic dye by titanium-based metal organic frameworks. J Chem Eng Data, 2017, 62(5): 1615 doi: 10.1021/acs.jced.6b01012 [22] Oveisi M, Asli M A, Mahmoodi N M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J Hazard Mater, 2018, 347: 123 doi: 10.1016/j.jhazmat.2017.12.057 [23] Fan Y H, Zhang S W, Qin S B, et al. An enhanced adsorption of organic dyes onto NH2 functionalization titanium-based metal-organic frameworks and the mechanism investigation. Microporous Mesoporous Mater, 2018, 263: 120 doi: 10.1016/j.micromeso.2017.12.016 [24] Li L, Liu X L, Geng H Y, et al. A MOF/graphite oxide hybrid (MOF: HKUST-1) material for the adsorption of methylene blue from aqueous solution. J Mater Chem A, 2013, 1(35): 10292 doi: 10.1039/c3ta11478c [25] Huang L J, He M, Chen B B, et al. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere, 2018, 199: 435 doi: 10.1016/j.chemosphere.2018.02.019 [26] Xu M J, Huang H T, Li N, et al. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol Environ Safety, 2019, 175: 289 doi: 10.1016/j.ecoenv.2019.01.131 [27] Wang J L, Zhuan R, Chu L B. The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview. Sci Total Environ, 2019, 646: 1385 doi: 10.1016/j.scitotenv.2018.07.415 [28] Azhar M R, Abid H R, Sun H Q, et al. Excellent performance of copper based metal organic framework in adsorptive removal of toxic sulfonamide antibiotics from wastewater. J Colloid Interface Sci, 2016, 478: 344 doi: 10.1016/j.jcis.2016.06.032 [29] Jiang J Q, Yang C X, Yan X P. Zeolitic imidazolate framework-8 for fast adsorption and removal of benzotriazoles from aqueous solution. ACS Appl Mater Interfaces, 2013, 5(19): 9837 doi: 10.1021/am403079n [30] Yang X Q, Yang C X, Yan X P. Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J Chromatogr A, 2013, 1304: 28 doi: 10.1016/j.chroma.2013.06.064 [31] Dehghan A, Zarei A, Jaafari J, et al. Tetracycline removal from aqueous solutions using zeolitic imidazolate frameworks with different morphologies: A mathematical modeling. Chemosphere, 2019, 217: 250 doi: 10.1016/j.chemosphere.2018.10.166 [32] Sun W L, Li H B, Li H M, et al. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: Batch experiment and DFT calculation. Chem Eng J, 2019, 360: 645 doi: 10.1016/j.cej.2018.12.021 [33] Hasan Z, Khan N A, Jhung S H. Adsorptive removal of diclofenac sodium from water with Zr-based metal-organic frameworks. Chem Eng J, 2016, 284: 1406 doi: 10.1016/j.cej.2015.08.087 [34] Zhuang S T, Cheng R, Wang J L. Adsorption of diclofenac from aqueous solution using UiO-66-type metal-organic frameworks. Chem Eng J, 2019, 359: 354 doi: 10.1016/j.cej.2018.11.150 [35] Liu W C, Shen X, Han Y Y, et al. Selective adsorption and removal of drug contaminants by using an extremely stable Cu(II)-based 3D metal-organic framework. Chemosphere, 2019, 215: 524 doi: 10.1016/j.chemosphere.2018.10.075 [36] Wang B, Lv X L, Feng D W, et al. Highly stable Zr(IV)-based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water. J Am Chem Soc, 2016, 138(19): 6204 doi: 10.1021/jacs.6b01663 [37] Song J Y, Jhung S H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: quantitative analyses of H-bonding in adsorption. Chem Eng J, 2017, 322: 366 doi: 10.1016/j.cej.2017.04.036 [38] Zhao X D, Zhao H F, Dai W J, et al. A metal-organic framework with large 1-D channels and rich -OH sites for high-efficiency chloramphenicol removal from water. J Colloid Interface Sci, 2018, 526: 28 doi: 10.1016/j.jcis.2018.04.095 [39] Zhuang S T, Liu Y, Wang J L. Mechanistic insight into the adsorption of diclofenac by MIL-100: Experiments and theoretical calculations. Environ Pollut, 2019, 253: 616 doi: 10.1016/j.envpol.2019.07.069 [40] Zhuo N, Lan Y Q, Yang W B, et al. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep Purif Technol, 2017, 177: 272 doi: 10.1016/j.seppur.2016.12.041 [41] Li Y X, Yang Z X, Wang Y L, et al. A mesoporous cationic thorium-organic framework that rapidly traps anionic persistent organic pollutants. Nature Commun, 2017, 8: 1354 doi: 10.1038/s41467-017-01208-w [42] Park E Y, Hasan Z, Khan N A, et al. Adsorptive removal of bisphenol-A from water with a metal-organic framework, a porous chromium-benzenedicarboxylate. J Nanosci Nanotechnol, 2013, 13(4): 2789 doi: 10.1166/jnn.2013.7411 [43] Liu B J, Yang F, Zou Y X, et al. Adsorption of phenol and p-nitrophenol from aqueous solutions on metal-organic frameworks: effect of hydrogen bonding. J Chem Eng Data, 2014, 59(5): 1476 doi: 10.1021/je4010239 [44] Sun B, Zhang L X, Yang L Z, et al. Agricultural non-point source pollution in China: causes and mitigation measures. Ambio, 2012, 41(4): 370 doi: 10.1007/s13280-012-0249-6 [45] Jung B K, Hasan Z, Jhung S H. Adsorptive removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from water with a metal-organic framework. Chem Eng J, 2013, 234: 99 doi: 10.1016/j.cej.2013.08.110 [46] Seo Y S, Khan N A, Jhung S H. Adsorptive removal of methylchlorophenoxypropionic acid from water with a metal-organic framework. Chem Eng J, 2015, 270: 22 doi: 10.1016/j.cej.2015.02.007 [47] Jia Y Y, Zhang Y H, Xu J, et al. A high-performance “sweeper” for toxic cationic herbicides: an anionic metal-organic framework with a tetrapodal cage. Chem Commun, 2015, 51(98): 17439 doi: 10.1039/C5CC07249B [48] Liu G Y, Li L Y, Xu D H, et al. Metal-organic framework preparation using magnetic graphene oxide-β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr Polym, 2017, 175: 584 doi: 10.1016/j.carbpol.2017.06.074 [49] Moeini Z, Azhdarpoor A, Yousefinejad S, et al. Removal of atrazine from water using titanium dioxide encapsulated in salicylaldehyde-NH2-MIL-101 (Cr): Adsorption or oxidation mechanism. J Clear Prod, 2019, 224: 238 doi: 10.1016/j.jclepro.2019.03.236 [50] Vermoortele F, Bueken B, Le Bars G, et al. Synthesis modulation as a tool to increase the catalytic activity of metal-organic frameworks: the unique case of UiO-66(Zr). J Am Chem Soc, 2013, 135(31): 11465 doi: 10.1021/ja405078u [51] Cai G R, Jiang H L. A Modulator-induced defect-formation strategy to hierarchically porous metal-organic frameworks with high stability. Angew Chem Int Ed, 2017, 56(2): 563 doi: 10.1002/anie.201610914 [52] Abdelhamid H N, Zou X D. Template-free and room temperature synthesis of hierarchical porous zeolitic imidazolate framework nanoparticles and their dye and CO2 sorption. Green chem, 2018, 20(5): 1074 doi: 10.1039/C7GC03805D [53] Tan J Y, Shi J X, Cui P H, et al. A Ni3(OH)(COO)6-based MOF from C3 symmetric ligands: Structure and heterogeneous catalytic activities in one-pot synthesis of imine. Microporous Mesoporous Mater, 2019, 287: 152 doi: 10.1016/j.micromeso.2019.06.003 [54] Gupta S S R, Kantam M L. Catalytic conversion of furfuryl alcohol or levulinic acid into alkyl levulinates using a sulfonic acid-functionalized hafnium-based MOF. Catal Commun, 2019, 124: 62 doi: 10.1016/j.catcom.2019.03.003 [55] Yang Y, Guo Z L, Chen X H, et al. A Ni3O-cluster based porous MOF for catalytic conversion of CO2 to cyclic carbonates. J Solid State Chem, 2019, 276: 190 doi: 10.1016/j.jssc.2019.05.010 [56] Zhang T Y, Liu W X, Meng G, et al. Construction of hierarchical copper-based metal-organic framework nanoarrays as functional structured catalysts. ChemCatChem, 2017, 9(10): 1771 doi: 10.1002/cctc.201700060 [57] Sun X Y, Shi Y, Zhang W, et al. A new type Ni-MOF catalyst with high stability for selective catalytic reduction of NOx with NH3. Catal Commun, 2018, 114: 104 doi: 10.1016/j.catcom.2018.06.012 [58] Chen X, Yu E Q, Cai S C, et al. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem Eng J, 2018, 344: 469 doi: 10.1016/j.cej.2018.03.091 [59] Gao Q, Xu J, Bu X H. Recent advances about metal-organic frameworks in the removal of pollutants from wastewater. Coordinat Chem Rev, 2019, 378: 17 doi: 10.1016/j.ccr.2018.03.015 [60] Wu Y, Luo H J, Wang H. Synthesis of iron (III)-based metal-organic framework/graphene oxide composites with increased photocatalytic performance for dye degradation. RSC Adv, 2014, 4(76): 40435 doi: 10.1039/C4RA07566H [61] Wang H, Yuan X Z, Wu Y, et al. In situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl Catal B-Environ, 2016, 186: 19 doi: 10.1016/j.apcatb.2015.12.041 [62] Wu X Y, Qi H X, Ning J J, et al. One silver(I)/tetraphosphine coordination polymer showing good catalytic performance in the photodegradation of nitroaromatics in aqueous solution. Appl Catal B-Environ, 2015, 168-169: 98 doi: 10.1016/j.apcatb.2014.12.024 [63] Wang D B, Jia F Y, Wang H, et al. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J Colloid Interface Sci, 2018, 519: 273 doi: 10.1016/j.jcis.2018.02.067 [64] Dong W B, Wang D B, Wang H, et al. Facile synthesis of In2S3/UiO-66 composite with enhanced adsorption performance and photocatalytic activity for the removal of tetracycline under visible light irradiation. J Colloid Interface Sci, 2019, 535: 444 doi: 10.1016/j.jcis.2018.10.008 [65] Rasheed H U, Lv X M, Zhang S Y, et al. Ternary MIL-100(Fe)@Fe3O4/CA magnetic nanophotocatalysts (MNPCs): Magnetically separable and Fenton-like degradation of tetracycline hydrochloride. Adv Powder Technol, 2018, 29(12): 3305 doi: 10.1016/j.apt.2018.09.011 [66] Mehrabadi Z, Faghihian H. Comparative photocatalytic performance of TiO2 supported on clinoptilolite and TiO2/Salicylaldehyde-NH2-MIL-101(Cr) for degradation of pharmaceutical pollutant atenolol under UV and visible irradiations. J Photochem Photobiol A, 2018, 356: 102 doi: 10.1016/j.jphotochem.2017.12.042 [67] Ren G B, Zhou M H, Liu M M, et al. A novel vertical-flow electro-Fenton reactor for organic wastewater treatment. Chem Eng J, 2016, 298: 55 doi: 10.1016/j.cej.2016.04.011 [68] Hu C J, Huang D L, Zeng G M, et al. The combination of Fenton process and Phanerochaete chrysosporium for the removal of bisphenol A in river sediments: mechanism related to extracellular enzyme, organic acid and iron. Chem Eng J, 2018, 338: 432 doi: 10.1016/j.cej.2018.01.068 [69] Cheng M, Zeng G M, Huang D L, et al. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J Hazard Mater, 2016, 312: 184 doi: 10.1016/j.jhazmat.2016.03.033 [70] Shao Z C, Huang C, Han X, et al. The effect of metal ions on photocatalytic performance based on an isostructural framework. Dalton Trans, 2015, 44(28): 12832 doi: 10.1039/C5DT01457C [71] Tang J T, Wang J L. Iron-copper bimetallic metal-organic frameworks for efficient Fenton-like degradation of sulfamethoxazole under mild conditions. Chemosphere, 2020, 241: 125002 doi: 10.1016/j.chemosphere.2019.125002 [72] Wu Q S, Yang H P, Kang L, et al. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(II)/Fe(III) cycle under visible light irradiation. Appl Catal B-Environ, 2020, 263: 118282 doi: 10.1016/j.apcatb.2019.118282 [73] Zhao H Y, Chen Y, Peng Q S, et al. Catalytic activity of MOF(2Fe/Co)/carbon aerogel for improving H2O2 and ·OH generation in solar photo-electro-Fenton process. Appl Catal B-Environ, 2017, 203: 127 doi: 10.1016/j.apcatb.2016.09.074 [74] Jia M Y, Yang Z H, Xu H Y, et al. Integrating N and F co-doped TiO2 nanotubes with ZIF-8 as photoelectrode for enhanced photo-electrocatalytic degradation of sulfamethazine. Chem Eng J, 2020, 388: 124388 doi: 10.1016/j.cej.2020.124388 [75] Xiong Y, Che L Y, Fu Z Y, et al. Preparation of CuxO/C composite derived from Cu-MOFs as Fenton-like catalyst by two-step calcination strategy. Adv Powder Technol, 2018, 29(6): 1331 doi: 10.1016/j.apt.2018.02.028 [76] Tang J T, Wang J L. MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation. Chem Eng J, 2019, 375: 122007 doi: 10.1016/j.cej.2019.122007 [77] Cashman M A, Kirschenbaum L, Holowachuk J, et al. Identification of hydroxyl and sulfate free radicals involved in the reaction of 1,4-dioxane with peroxone activated persulfate oxidant. J Hazard Mater, 2019, 380: 120875 doi: 10.1016/j.jhazmat.2019.120875 [78] Yun W C, Lin K Y A, Tong W C, et al. Enhanced degradation of paracetamol in water using sulfate radical-based advanced oxidation processes catalyzed by 3-dimensional Co3O4 nanoflower. Chem Eng J, 2019, 373: 1329 doi: 10.1016/j.cej.2019.05.142 [79] Ke Q, Shi Y P, Liu Y X, et al. Enhanced catalytic degradation of bisphenol A by hemin-MOFs supported on boron nitride via the photo-assisted heterogeneous activation of persulfate. Sep Purif Technol, 2019, 229: 115822 doi: 10.1016/j.seppur.2019.115822 [80] Azhar M R, Vijay P, Tadé M O, et al. Submicron sized water-stable metal organic framework (bio-MOF-11) for catalytic degradation of pharmaceuticals and personal care products. Chemosphere, 2018, 196: 105 doi: 10.1016/j.chemosphere.2017.12.164 [81] Zhang S W, Gao H H, Xu X T, et al. MOF-derived CoN/N-C@SiO2 yolk-shell nanoreactor with dual active sites for highly efficient catalytic advanced oxidation processes. Chem Eng J, 2020, 381: 122670 doi: 10.1016/j.cej.2019.122670 [82] Zeng T, Zhang X L, Wang S H, et al. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ Sci Technol, 2015, 49(4): 2350 doi: 10.1021/es505014z [83] Li X H, Guo W L, Liu Z H, et al. Quinone-modified NH2-MIL-101(Fe) composite as a redox mediator for improved degradation of bisphenol A. J Hazard Mater, 2017, 324: 665 doi: 10.1016/j.jhazmat.2016.11.040 [84] Fang Z L, Bueken B, De Vos D E, et al. Defect-engineered metal-organic frameworks. Angew Chem Int Ed, 2015, 54(25): 7234 doi: 10.1002/anie.201411540 [85] Szilágyi P á, Serra-Crespo P, Gascon J, et al. The impact of post-synthetic linker functionalization of MOFs on methane storage: The role of defects. Front Energy Res, 2016, 4: 9 [86] Li J, Liu Y, Wang X X, et al. Experimental and theoretical study on selenate uptake to zirconium metal-organic frameworks: Effect of defects and ligands. Chem Eng J, 2017, 330: 1012 doi: 10.1016/j.cej.2017.08.038 [87] Jiao Y, Liu Y, Zhu G H, et al. Heat-treatment of defective UiO-66 from modulated synthesis: adsorption and stability studies. J Phys Chem C, 2017, 121(42): 23471 doi: 10.1021/acs.jpcc.7b07772 [88] Plonka A M, Wang Q, Gordon W O, et al. In situ probes of capture and decomposition of chemical warfare agent simulants by Zr-based metal organic frameworks. J Am Chem Soc, 2017, 139(2): 599 doi: 10.1021/jacs.6b11373 [89] De Vos A, Hendrickx K, Van Der Voort P, et al. Missing linkers: an alternative pathway to UiO-66 electronic structure engineering. Chem Mater, 2017, 29(7): 3006 doi: 10.1021/acs.chemmater.6b05444 [90] Cao J, Yang Z H, Xiong W P, et al. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem Eng J, 2018, 353: 126 doi: 10.1016/j.cej.2018.07.060 [91] Fan K, Nie W X, Wang L P, et al. Defective metal-organic frameworks incorporating iridium-based metalloligands: Sorption and dye degradation properties. Chem Eur J, 2017, 23(27): 6615 doi: 10.1002/chem.201700365 [92] Mondloch J E, Katz M J, Isley III W C, et al. Destruction of chemical warfare agents using metal-organic frameworks. Nature Mater, 2015, 14(5): 512 doi: 10.1038/nmat4238 [93] Cho K Y, Seo J Y, Kim H J, et al. Facile control of defect site density and particle size of UiO-66 for enhanced hydrolysis rates: insights into feasibility of Zr(IV)-based metal-organic framework (MOF) catalysts. Appl Catal B-Environ, 2019, 245: 635 doi: 10.1016/j.apcatb.2019.01.033 [94] C?té A P, Benin A I, Ockwig N W, et al. Porous, crystalline, covalent organic frameworks. Science, 2005, 310(5751): 1166 doi: 10.1126/science.1120411 [95] Ding S Y, Gao J, Wang Q, et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU-1 in Suzuki-Miyaura coupling reaction. J Am Chem Soc, 2011, 133(49): 19816 doi: 10.1021/ja206846p [96] Guan X Y, Li H, Ma Y C, et al. Chemically stable polyarylether-based covalent organic frameworks. Nature Chem, 2019, 11(6): 587 doi: 10.1038/s41557-019-0238-5 -




 下載:
下載: