Research progress on the preparation and corrosion resistance of layered double hydroxides film on aluminum alloys
-
摘要: 鋁合金具有密度小,比強度高等一系列優異的性能而受到研究者的關注,但其易腐蝕的特點嚴重制約了其應用范圍,因此需要采取適當的方法增強其耐蝕性能。水滑石薄膜具有良好的耐蝕性與離子交換性能,近年來在鋁合金表面改性技術的研究逐漸增多。本文介紹了多種制備水滑石薄膜的方法,探究不同實驗條件對薄膜形貌與耐蝕性的影響;詳述了幾種常用的改性方法與原理,對目前研究中存在的局限性進行了討論,并展望了未來研究的重點與發展方向。Abstract: Aluminum alloys have excellent properties such as low density and high strength-to-weight ratio. However, the negative standard electrode potential of aluminum leads to a more active chemical property and is prone to corrode; as a result, the poor corrosion resistance extremely limits the widespread application of aluminum. Therefore, it is necessary to take appropriate measures to improve the poor corrosion resistance of aluminum alloys. The chromate passivation technology is one of the most effective and mature aluminum alloy surface treatment technologies, and even if the formed passivation film is very thin, it can still greatly enhance the corrosion resistance of aluminum alloys and provide corrosion protection. However, Cr (VI) and its derivatives are highly toxic and carcinogenic, and they are harmful to the environment and the human body. As environmental awareness increases and the government strictly limits the use and emission of chromate, it is necessary to develop new treatments that are environmentally friendly and non-toxic to improve the corrosion resistance of aluminum alloys. The fabrication process of layered double hydroxides (LDHs) film is simple, and the morphology of the LDHs film can be controlled by adjusting the experimental parameters. The prepared LDHs film also has good corrosion resistance and anions exchange performance. Therefore, reports of in-situ growth LDHs film on the surface aluminum alloys have gradually increased in recent years. In this paper, we introduced a variety of methods for preparing LDHs film, such as ordinary hydrothermal, urea hydrolysis, and hexamethylenetetramine hydrolysis methods, and summarized the effects of different experimental conditions on the morphology and corrosion resistance of LDHs the films. Several commonly used modification methods and principles, such as the preparation of superhydrophobic films and self-healing films, were discussed in detail and the limitations of the current research were discussed. Finally, the focus of future research and development were described.
-
Key words:
- aluminum alloys /
- layered double hydroxides /
- surface modification /
- corrosion resistance /
- in-situ grow
-
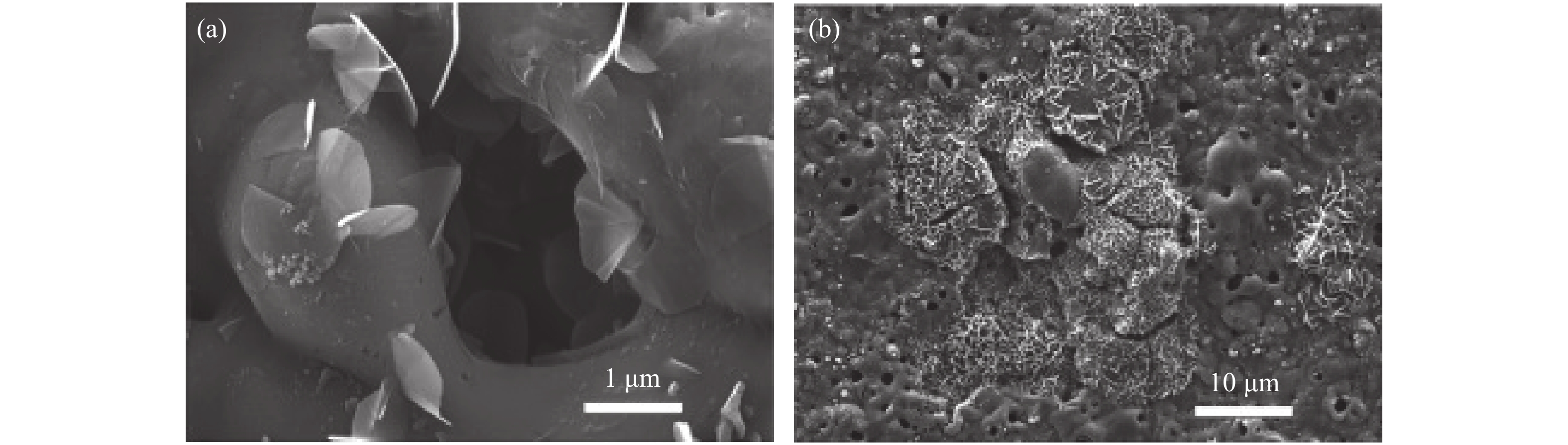
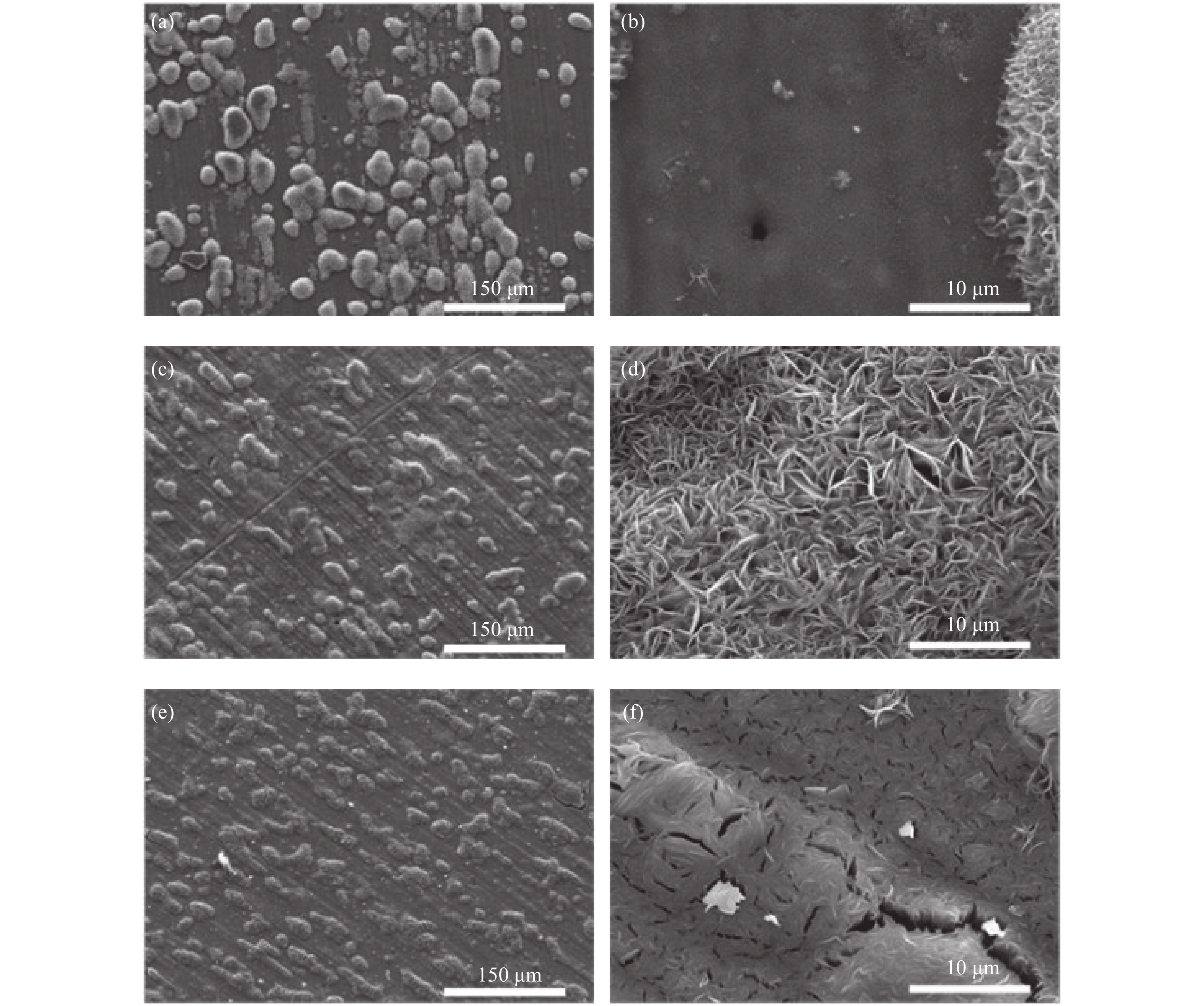
圖 1 不同Zn2+濃度條件下在AA2024鋁合金基體制備的Zn?Al水滑石薄膜的掃描電鏡圖. (a,b) 5 mmol?L?1 Zn2+;(c,d) 50 mmol?L?1 Zn2+;(e,f) 500 mmol?L?1 Zn2+
Figure 1. SEM images of AA2024-T3 substrates covered with Zn?Al LDHs thin film prepared under different Zn2+ concentrations: (a,b) 5 mmol?L?1 Zn2+; (c,d) 50 mmol?L?1 Zn2+; (e,f) 500 mmol?L?1 Zn2+
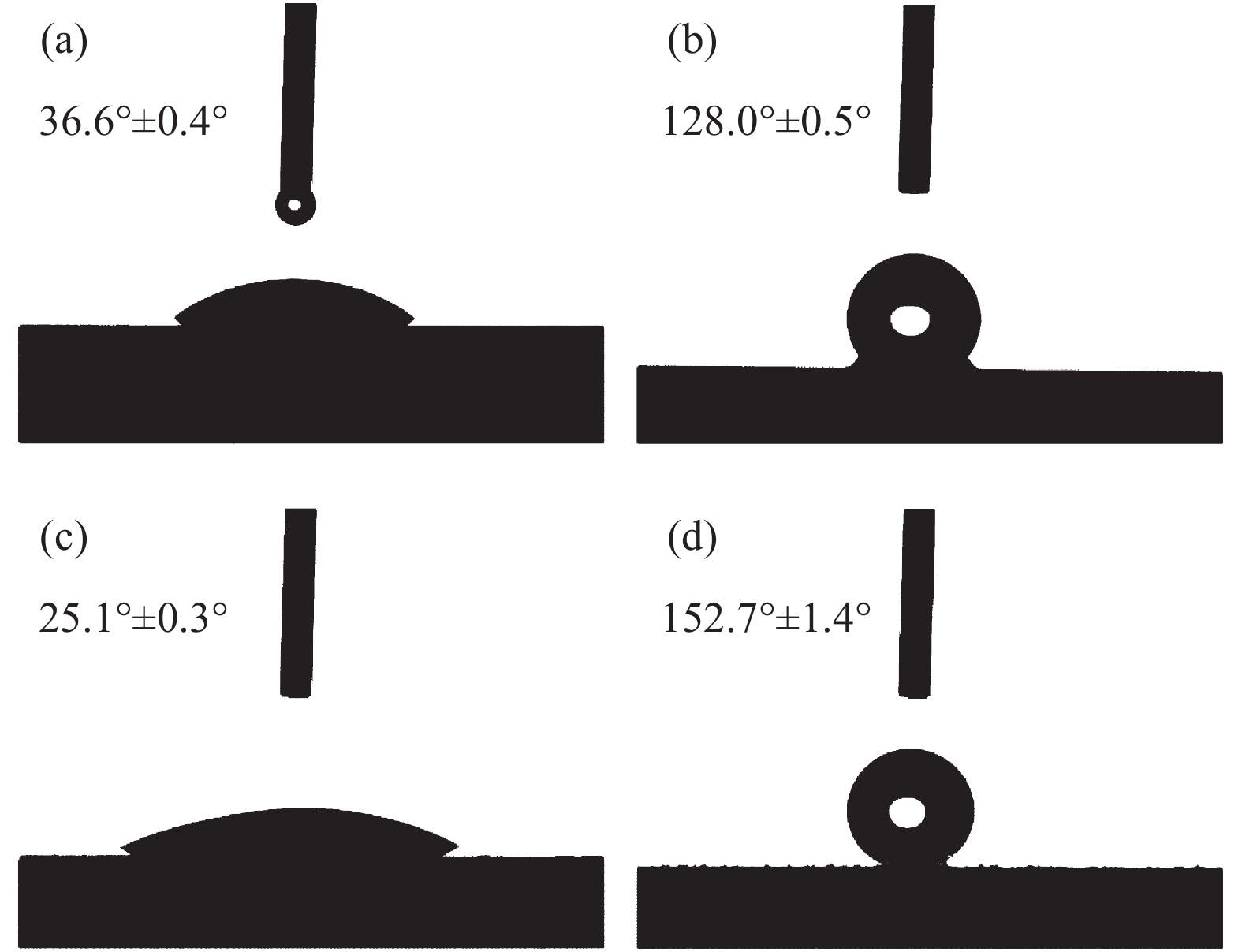
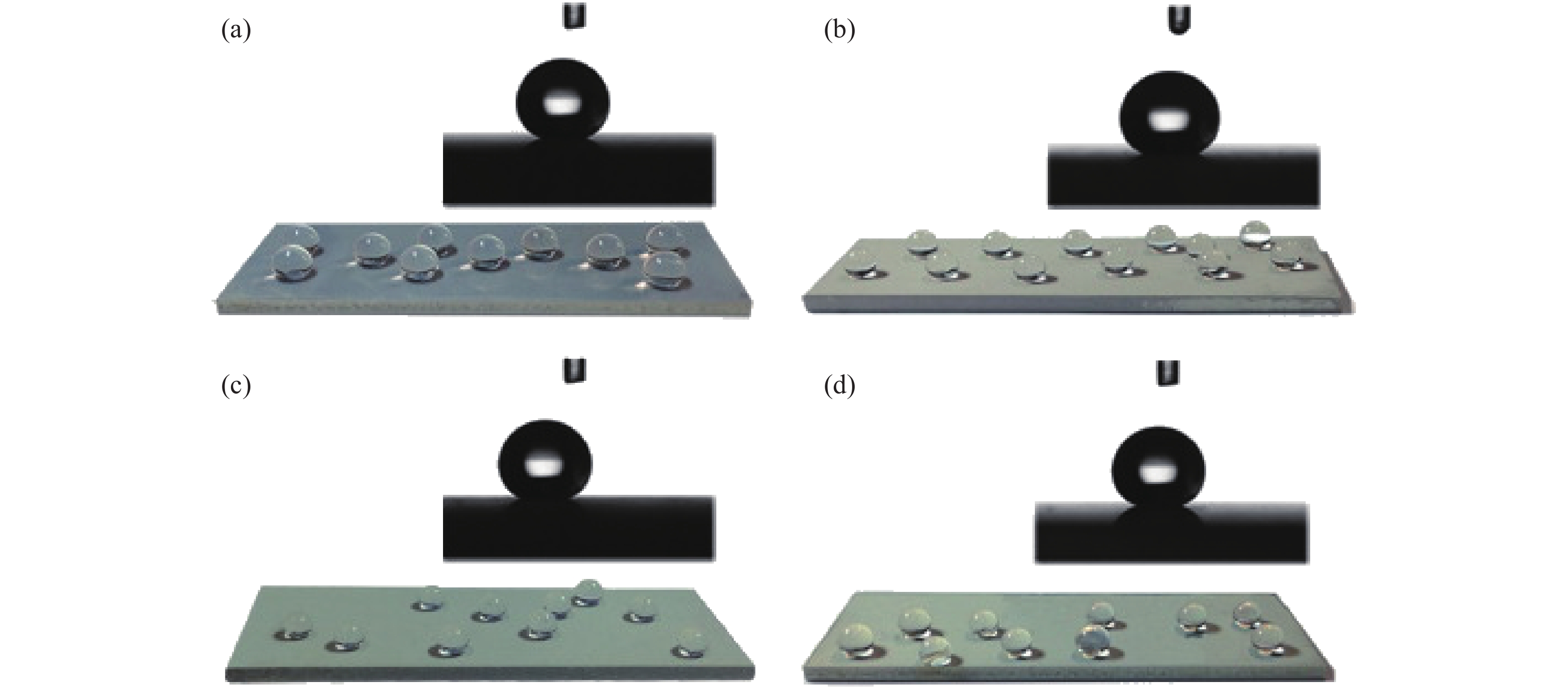
圖 6 不同水滑石薄膜樣品用1H,1H,2H,2H-全氟癸基三甲氧基硅烷進行表面改性后的接觸角與對應水滴照片。(a) Mg?Al水滑石,接觸角為168.8°; (b) Co?Al水滑石,接觸角為169.6°;(c) Ni?Al水滑石,接觸角為165.8°;(d) Zn?Al水滑石,接觸角為164.2°
Figure 6. CA of different LDHs thin film samples with surface modification with PFDTMS and the corresponding photographs of water droplets on the surfaces: (a) Mg?Al LDHs, CA=168.8°; (b) Co?Al LDHs, CA=169.6°; (c) Ni?Al LDHs, CA=165.8°; (d) Zn?Al LDHs, CA=164.2°
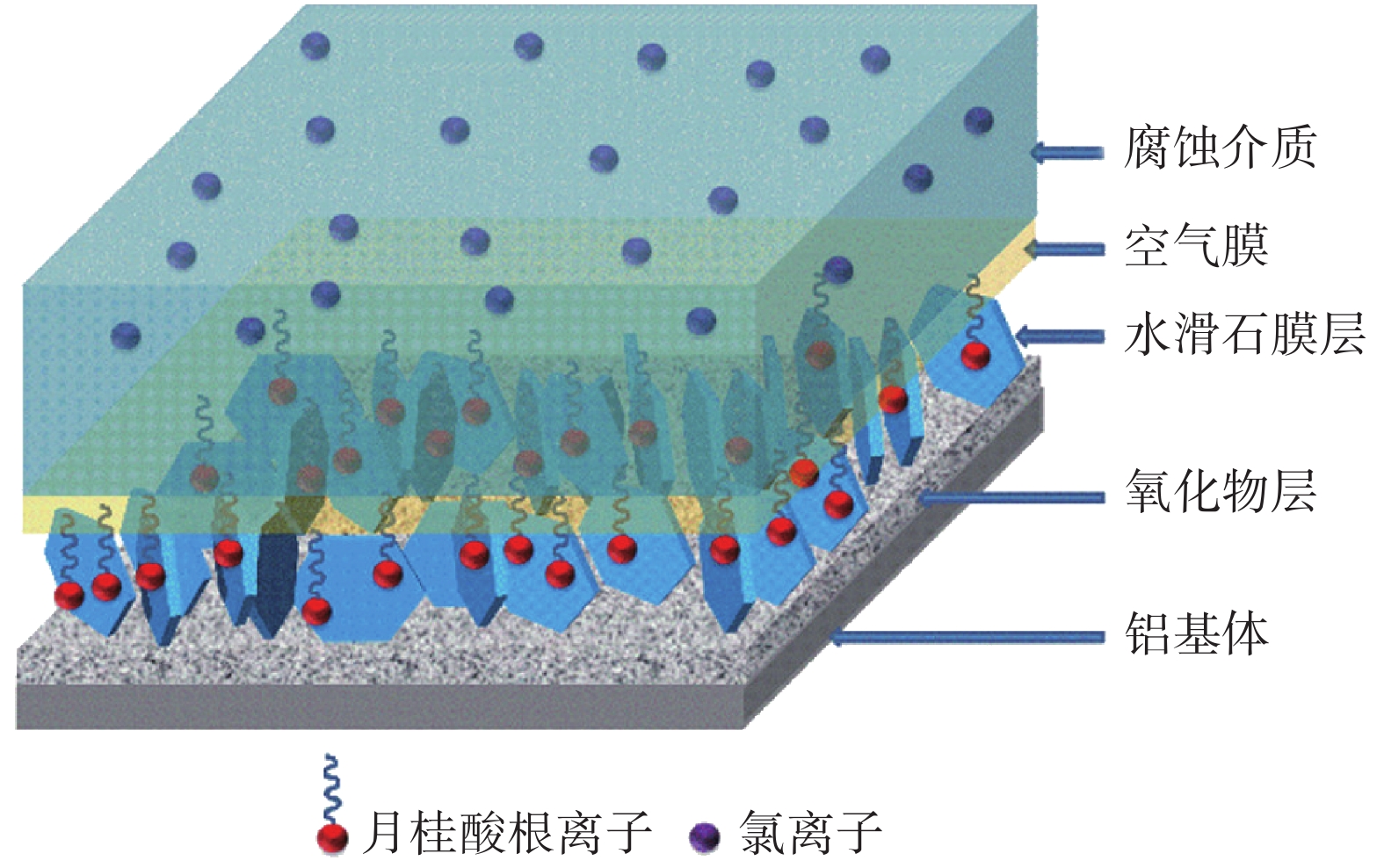
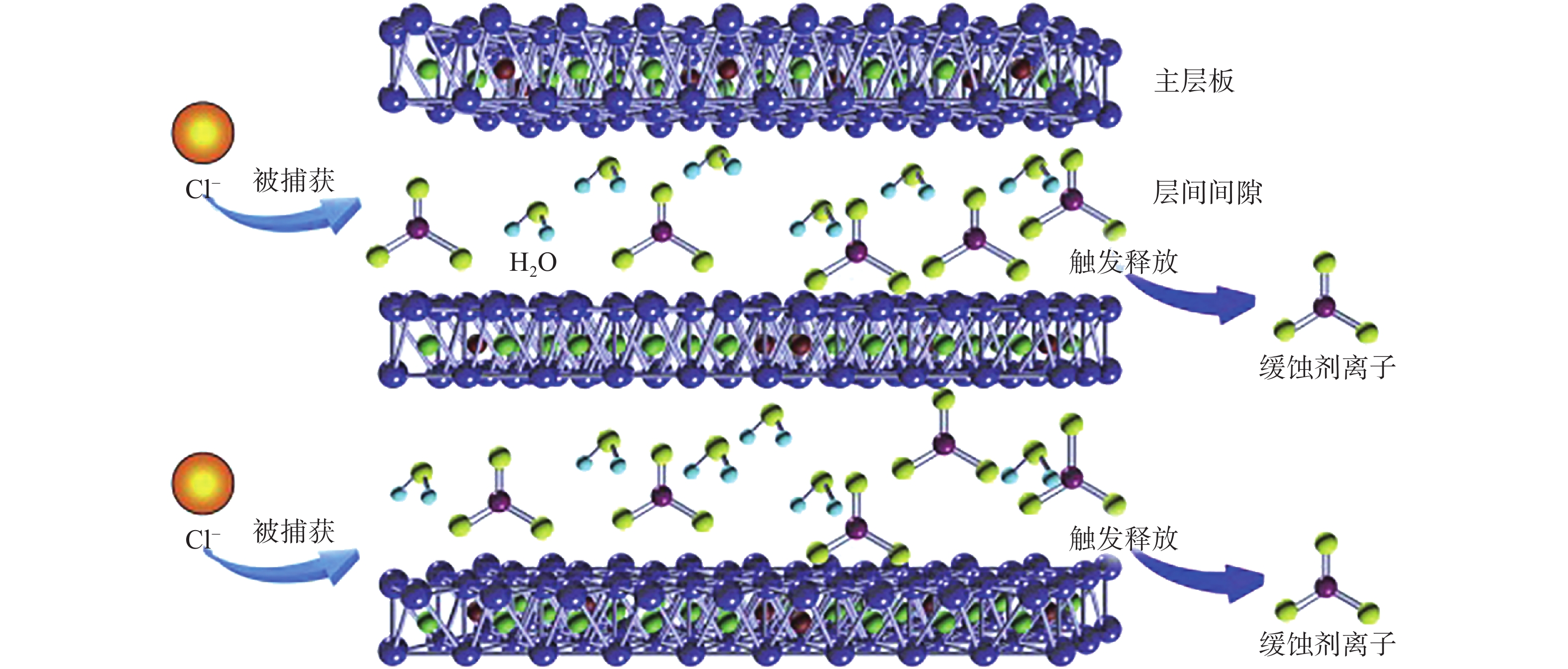
圖 7 水滑石薄膜捕獲Cl?與釋放緩蝕劑的示意圖[9]
Figure 7. Schematic representation of the entrapment Cl? and the triggered release of anionic corrosion inhibitors from LDHs
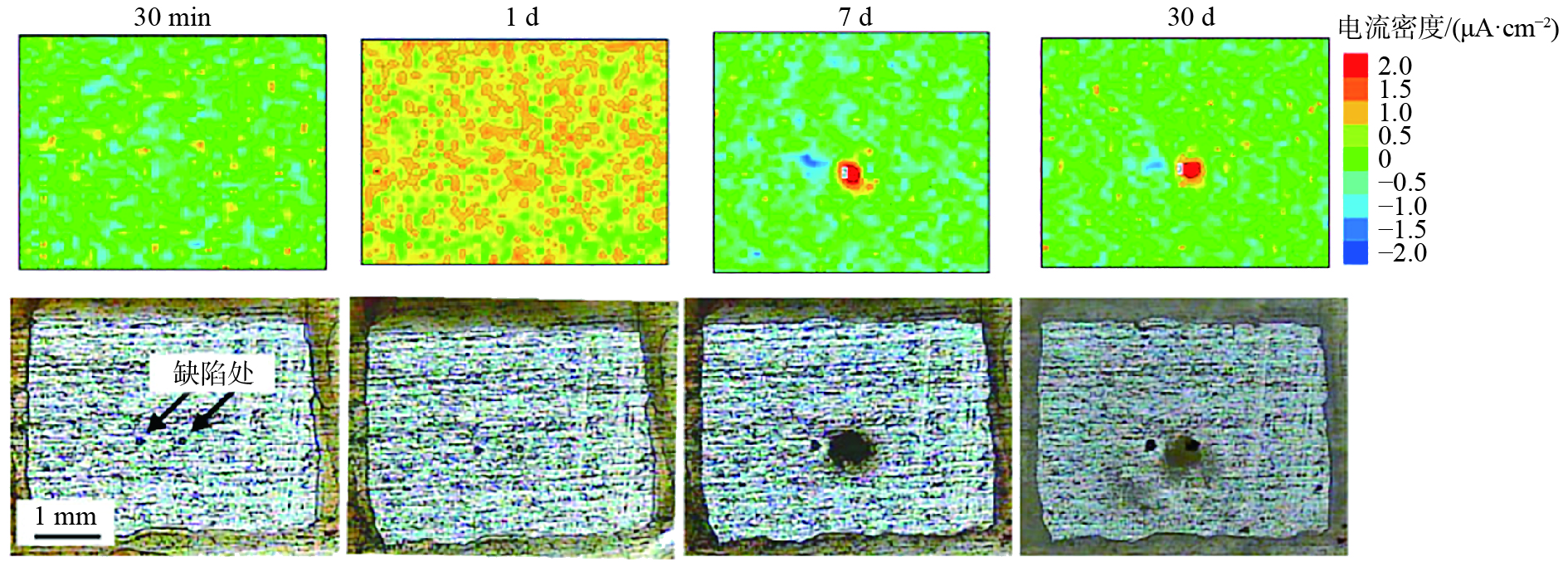
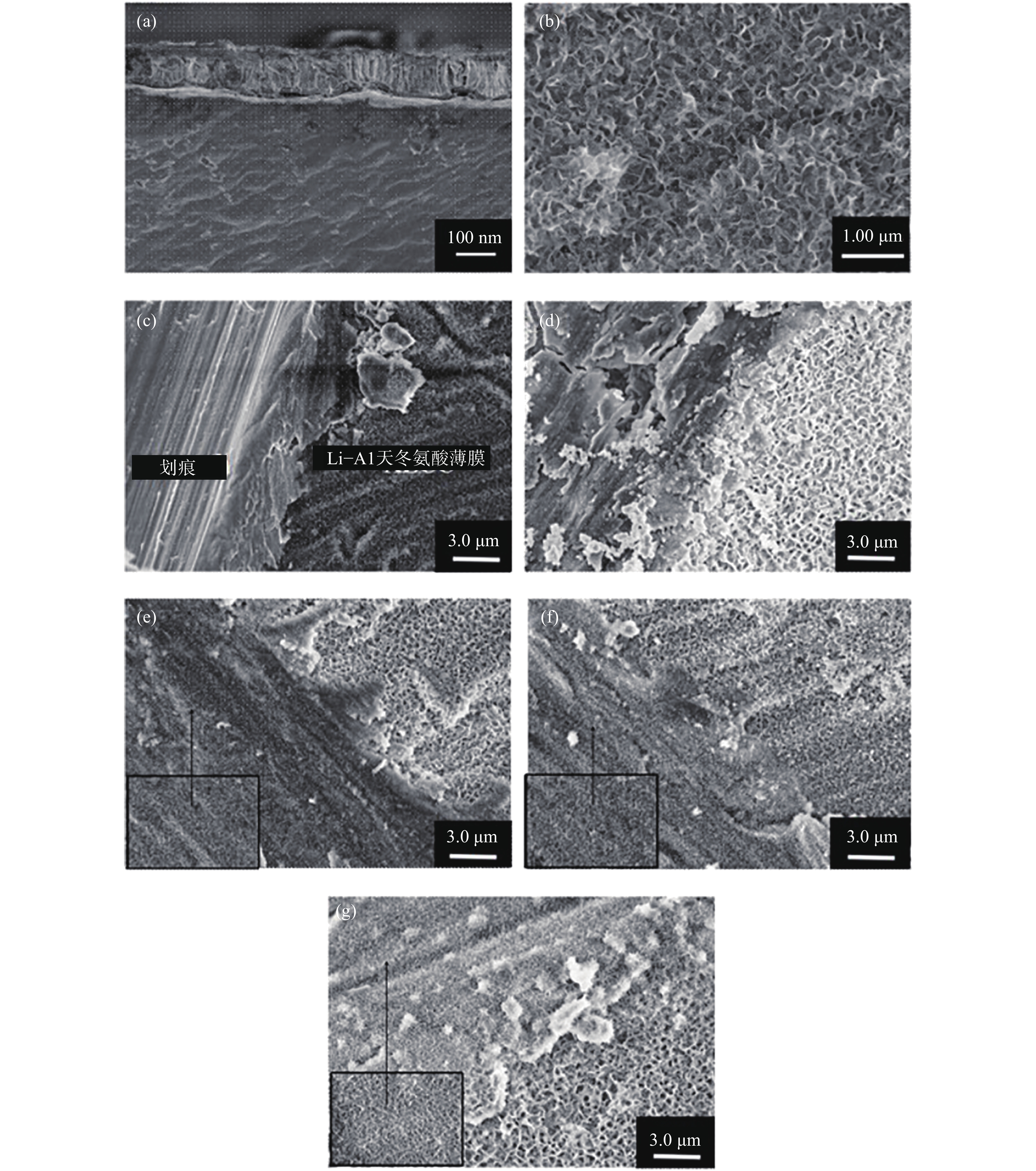
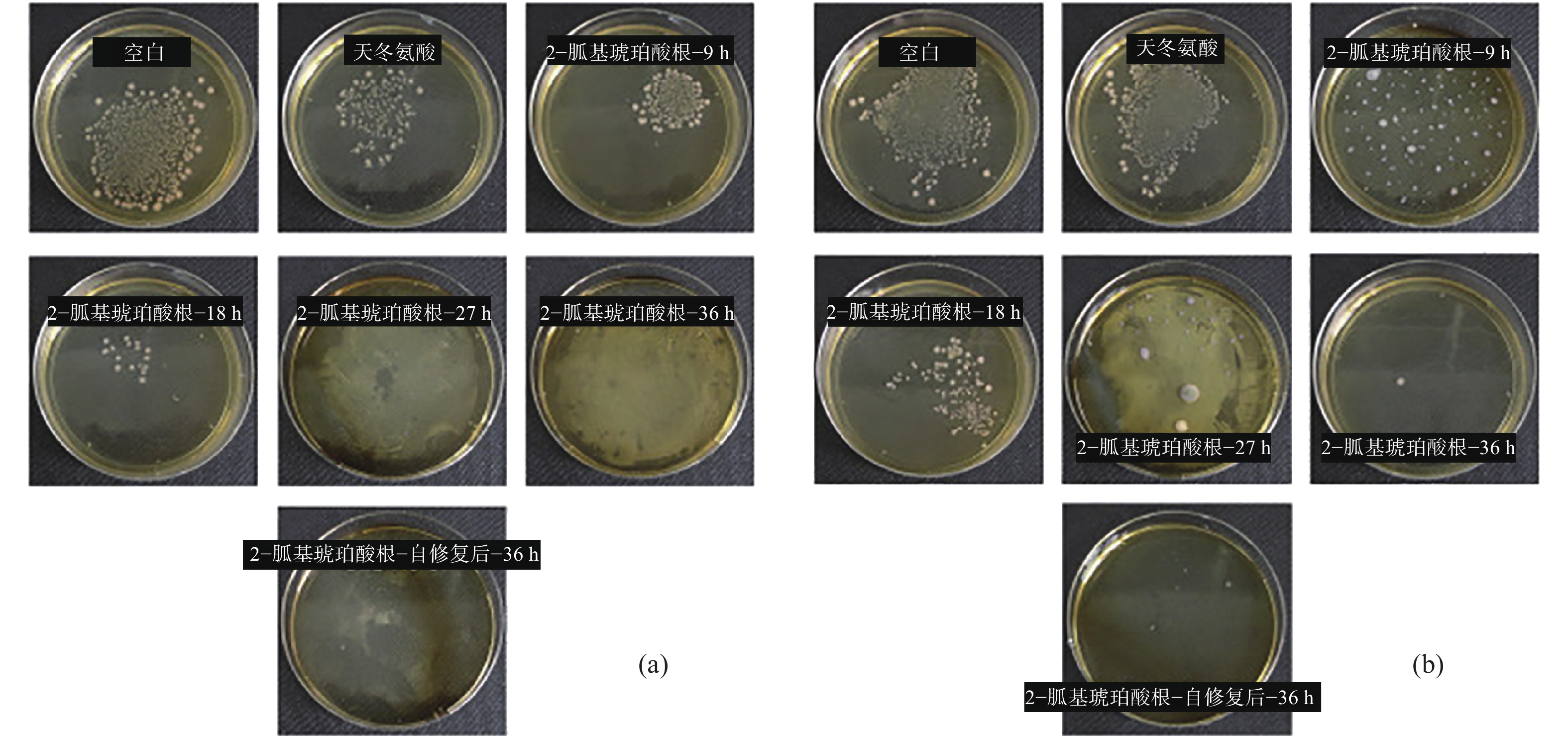
圖 9 Li?Al天冬氨酸水滑石薄膜微觀形貌分析。(a) 截面形貌;(b) 表面形貌;具有人工劃痕的Li?Al天冬氨酸水滑石薄膜在3.5%(質量分數)NaCl溶液浸泡不同時間后的形貌,(c) 0;(d) 2 d;(e) 6 d;(f) 9 d;(g) 20 d
Figure 9. SEM images of Li?Al?Asp LDHs: (a) cross-section; (b) surface; SEM images of Li?Al?Asp LDHs with artificial scratch after immersion in 3.5% (mass fraction) NaCl solution for different times: (c) 0; (d) 2 d; (e) 6 d; (f) 9 d; (g) 20 d
259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Zhang Y L, Yu P H, Wei Y H, et al. Influence of graphene additive on wear resistance and corrosion resistance of micro arc oxidation coating formed on LY12 aluminium alloy surface. Trans Mater Heat Treat, 2017, 38(8): 103張玉林, 于佩航, 韋銀河, 等. 石墨烯添加劑對鋁合金表面微弧氧化膜耐磨耐蝕性的影響. 材料熱處理學報, 2017, 38(8):103 [2] Laurino A, Andrieu E, Harouard J P, et al. Effect of corrosion on the fatigue life and fracture mechanisms of 6101 aluminum alloy wires for car manufacturing applications. Mater Des, 2014, 53: 236 doi: 10.1016/j.matdes.2013.06.079 [3] Zhang Y S, Wang Y B, Li C M, et al. Preparation and corrosion resistance of the ZnAl?LDHs film on 6061 Al alloy surface. Acta Metall Sin, 2018, 54(10): 1417 doi: 10.11900/0412.1961.2018.00020張玉圣, 王友彬, 李純民, 等. 6061鋁合金表面ZnAl?LDHs層的制備及其耐腐蝕性能. 金屬學報, 2018, 54(10):1417 doi: 10.11900/0412.1961.2018.00020 [4] Nie M, Huang F, Wang Z G, et al. Electrochemical synthesis of polypyrrole/polydopamine for aluminum alloy corrosion inhibition. Acta Mater Compos Sin, 2018, 36(10): 2364聶銘, 黃豐, 王珍高, 等. 聚吡咯/聚多巴胺的電化學合成及其對鋁合金的耐蝕性影響. 復合材料學報, 2018, 36(10):2364 [5] Xu Z S, Zhou Q, Zhu Z T, et al. Effects of pulse frequency on microstructure and corrosion resistance of micro-arc oxidation coating on A7N01P-T4 aluminum alloy. Hot Work Technol, 2019, 48(14): 112徐袛尚, 周強, 朱宗濤, 等. 脈沖頻率對A7N01P-T4鋁合金微弧氧化膜微觀結構和耐蝕性的影響. 熱加工工藝, 2019, 48(14):112 [6] Tedim J, Zheludkevich M L, Bastos A C, et al. Effect of surface treatment on the performance of LDH conversion films. ECS Electrochem Lett, 2014, 3(1): C4 [7] Cao Y H, Zheng D J, Li X L, et al. Enhanced corrosion resistance of superhydrophobic layered double hydroxide films with long-term stability on Al substrate. ACS Appl Mater Interfaces, 2018, 10(17): 15150 doi: 10.1021/acsami.8b02280 [8] Guo L, Wu W, Zhou Y F, et al. Layered double hydroxide coatings on magnesium alloys: a review. J Mater Sci Technol, 2018, 34(9): 1455 doi: 10.1016/j.jmst.2018.03.003 [9] Zhang G, Wu L, Tang A T, et al. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros Sci, 2018, 139: 370 doi: 10.1016/j.corsci.2018.05.010 [10] Gong M, Li Y G, Wang H L, et al. An advanced Ni?Fe layered double hydroxide electrocatalyst for water oxidation. J Am Chem Soc, 2013, 135(23): 8452 doi: 10.1021/ja4027715 [11] Song F, Hu X L. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat Commun, 2014, 5: 4477 doi: 10.1038/ncomms5477 [12] Liu Y P, Liang X, Gu L, et al. Corrosion engineering towards efficient oxygen evolution electrodes with stable catalytic activity for over 6000 hours. Nat Commun, 2018, 9: 2609 doi: 10.1038/s41467-018-05019-5 [13] Tang P G, Feng Y J, Li D Q. Fabrication and properties of acid blue 25 dye?intercalated layered double hydroxides film on an anodic alumina/aluminum substrate. J Phys Chem Solids, 2012, 73(12): 1505 doi: 10.1016/j.jpcs.2011.12.027 [14] Tang P G, Feng Y J, Li D Q. Fabrication and properties of Acid Yellow 49 dye?intercalated layered double hydroxides film on an alumina-coated aluminum substrate. Dyes Pigm, 2011, 91(2): 120 doi: 10.1016/j.dyepig.2011.03.012 [15] Zhang C X, Luo X H, Pan X Y, et al. Self-healing Li?Al layered double hydroxide conversion coating modified with aspartic acid for 6N01 Al alloy. Appl Surf Sci, 2017, 394: 275 doi: 10.1016/j.apsusc.2016.10.034 [16] Ba Z X, Chen Y J, Dong Q S, et al. Research progress on corrosion resistance of hydrotalcite films on magnesium alloys. Mater Rev, 2017, 31(6): 144巴志新, 陳永俊, 董強勝, 等. 鎂合金表面水滑石膜耐蝕性研究進展. 材料導報, 2017, 31(6):144 [17] Wang F Y, Guo Z G. In-situ growth of durable superhydrophobic Mg?Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J Alloys Compd, 2018, 767: 382 doi: 10.1016/j.jallcom.2018.07.086 [18] Tedim J, Zheludkevich M L, Salak A N, et al. Nanostructured LDH-container layer with active protection functionality. J Mater Chem, 2011, 21(39): 15464 doi: 10.1039/c1jm12463c [19] Li Y D, Li S M, Zhang Y, et al. Enhanced protective Zn?Al layered double hydroxide film fabricated on anodized 2198 aluminum alloy. J Alloys Compd, 2015, 630: 29 doi: 10.1016/j.jallcom.2014.12.176 [20] Kaseem M, Ko Y G. Benzoate intercalated Mg?Al?ayered double hydroxides (LDHs) as efficient chloride traps for plasma electrolysis coatings. J Alloys Compd, 2019, 787: 772 doi: 10.1016/j.jallcom.2019.02.124 [21] Guo Y G, Wang Q H, Wang T M. Facile fabrication of superhydrophobic surface with micro/nanoscale binary structures on aluminum substrate. Appl Surf Sci, 2011, 257(13): 5831 doi: 10.1016/j.apsusc.2011.01.114 [22] Li W, Zhang X, Yang J, et al. In situ growth of superhydrophobic and icephobic films with micro/nanoscale hierarchical structures on the aluminum substrate. J Colloid Interface Sci, 2013, 410: 165 doi: 10.1016/j.jcis.2013.07.063 [23] Xu Z P, Lu G Q. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3 LDH formation mechanism. Chem Mater, 2005, 17(5): 1055 doi: 10.1021/cm048085g [24] Iqbal M A, Fedel M. The effect of the surface morphologies on the corrosion resistance of in situ growth MgAl?LDH based conversion film on AA6082. Surf Coat Technol, 2018, 352: 166 doi: 10.1016/j.surfcoat.2018.08.006 [25] Tedim J, Zheludkevich M L, Bastos A C, et al. Influence of preparation conditions of layered double hydroxide conversion films on corrosion protection. Electrochim Acta, 2014, 117: 164 doi: 10.1016/j.electacta.2013.11.111 [26] Zhang Y, Liu J H, Li Y D, et al. A facile approach to superhydrophobic LiAl?layered double hydroxide film on Al?Li alloy substrate. J Coat Technol Res, 2015, 12(3): 595 doi: 10.1007/s11998-015-9660-9 [27] Chen F, Yu P H, Zhang Y. Healing effects of LDHs nanoplatelets on MAO ceramic layer of aluminum alloy. J Alloys Compd, 2017, 711: 342 doi: 10.1016/j.jallcom.2017.04.016 [28] Lü Z, Zhang F Z, Lei X D, et al. In situ growth of layered double hydroxide films on anodic aluminum oxide/aluminum and its catalytic feature in aldol condensation of acetone. Chem Eng Sci, 2008, 63(16): 4055 doi: 10.1016/j.ces.2008.05.007 [29] Zhang Y, Li Y D, Ren Y S, et al. Double-doped LDH films on aluminum alloys for active protection. Mater Lett, 2017, 192: 33 doi: 10.1016/j.matlet.2017.01.038 [30] Wang Y, Zhang D, Lu Z. Hydrophobic Mg?Al layered double hydroxide film on aluminum: Fabrication and microbiologically influenced corrosion resistance properties. Colloids Surf A, 2015, 474: 44 doi: 10.1016/j.colsurfa.2015.03.005 [31] Wu J S, Peng D D, He Y T, et al. In-situ formation of decavanadate-intercalated layered double hydroxide films on AA2024 and their anti-corrosive properties when combined with hybrid sol gel films. Materials, 2017, 10(4): 426 doi: 10.3390/ma10040426 [32] Kaseem M, Ko Y G. A novel composite system composed of zirconia and LDHs film grown on plasma electrolysis coating: Toward a stable smart coating. Ultrason Sonochem, 2018, 49: 316 doi: 10.1016/j.ultsonch.2018.08.023 [33] Lv Y Z, Wang L F, Ma K B, et al. Fabrication of superhydrophobic films on aluminum foils with controllable morphologies. Adv Mater Res, 2013, 641-642: 414 doi: 10.4028/www.scientific.net/AMR.641-642.414 [34] Iyi N, Matsumoto T, Kaneko Y, et al. Deintercalation of carbonate ions from a hydrotalcite-like compound: Enhanced decarbonation using acid-salt mixed solution. Chem Mater, 2004, 16(15): 2926 doi: 10.1021/cm049579g [35] Zhang T, Yu H Q, Zhou Y M, et al. In situ fabrication and infrared emissivity properties of oriented LDHs films on Al substrates. RSC Adv, 2015, 5(100): 82415 doi: 10.1039/C5RA15962H [36] Scarpellini D, Falconi C, Gaudio P, et al. Morphology of Zn/Al layered double hydroxide nanosheets grown onto aluminum thin films. Microelectron Eng, 2014, 126: 129 doi: 10.1016/j.mee.2014.07.007 [37] Yarger M S, Steinmiller E M P, Choi K S. Electrochemical synthesis of Zn?Al layered double hydroxide (LDH) films. Inorg Chem, 2008, 47(13): 5859 doi: 10.1021/ic800193j [38] Scavetta E, Mignani A, Prandstraller D, et al. Electrosynthesis of thin films of Ni, Al hydrotalcite like compounds. Chem Mater, 2007, 19(18): 4523 doi: 10.1021/cm071132v [39] Zhang Y, Liu J H, Li Y D, et al. Enhancement of active anticorrosion via Ce-doped Zn?Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy. J Wuhan Univ Technol Mater Sci Ed, 2017, 32(5): 1199 doi: 10.1007/s11595-017-1731-6 [40] Liu J H, Zhang Y, Yu M, et al. Influence of embedded ZnAlCe? $\scriptstyle{\rm{NO}}^{-}_{3}$ layered double hydroxides on the anticorrosion properties of sol-gel coatings for aluminum alloy. Prog Org Coat, 2015, 81: 93 doi: 10.1016/j.porgcoat.2014.12.015[41] Shan D, Cosnier S, Mousty C. Layered double hydroxides: an attractive material for electrochemical biosensor design. Anal Chem, 2003, 75(15): 3872 doi: 10.1021/ac030030v [42] Barhoumi H, Maaref A, Rammah M, et al. Urea biosensor based on Zn3Al?Urease layered double hydroxides nanohybrid coated on insulated silicon structures. Mater Sci Eng C, 2006, 26(2-3): 328 doi: 10.1016/j.msec.2005.10.042 [43] Indira L, Dixit M, Kamath P V. Electrosynthesis of layered double hydroxides of nickel with trivalent cations. J Power Sources, 1994, 52(1): 93 doi: 10.1016/0378-7753(94)01939-8 [44] Roto R, Yamagishi A, Villemure G. Electrochemical quartz crystal microbalance study of mass transport in thin film of a redox active Ni?Al?Cl layered double hydroxide. J Electroanal Chem, 2004, 572(1): 101 doi: 10.1016/j.jelechem.2004.06.005 [45] Zhang F Z, Sun M, Xu S L, et al. Fabrication of oriented layered double hydroxide films by spin coating and their use in corrosion protection. Chem Eng J, 2008, 141(1-3): 362 doi: 10.1016/j.cej.2008.03.016 [46] De D, Sarkar D K. Superhydrophobic ZnAl double hydroxide nanostructures and ZnO films on Al and glass substrates. Mater Chem Phys, 2017, 185: 195 doi: 10.1016/j.matchemphys.2016.10.022 [47] Chen H, Zhang F, Fu S, et al. In situ microstructure control of oriented layered double hydroxide monolayer films with curved hexagonal crystals as superhydrophobic materials. Adv Mater, 2006, 18(23): 3089 doi: 10.1002/adma.200600615 [48] Yue C L, Chen H Y, Xu S L, et al. Controlled synthesis and investigation of the mechanism of formation of hollow hemispherical protrusions on laurate anion-intercalated Zn/Al layered double hydroxide hybrid films. J Colloid Interface Sci, 2012, 385(1): 268 doi: 10.1016/j.jcis.2012.06.008 [49] Zhang Y, Yu P H, Wang J P, et al. LDHs/graphene film on aluminum alloys for active protection. Appl Surf Sci, 2018, 433: 927 doi: 10.1016/j.apsusc.2017.10.126 [50] Li Y D, Li S M, Zhang Y, et al. Fabrication of superhydrophobic layered double hydroxides films with different metal cations on anodized aluminum 2198 alloy. Mater Lett, 2015, 142: 137 doi: 10.1016/j.matlet.2014.11.148 [51] Liu D, Song Y W, Shan D Y, et al. Self-healing coatings form agnesium alloys: a review. Surf Technol, 2016, 45(12): 28劉丹, 宋影偉, 單大勇, 等. 鎂合金自修復涂層研究進展. 表面技術, 2016, 45(12):28 [52] Pan M Q, Wang L T, Ding X, et al. The research progress of self-healing anti-corrosion coatings. Mater China, 2018, 37(1): 19潘夢秋, 王倫滔, 丁璇, 等. 自修復防腐涂層研究進展. 中國材料進展, 2018, 37(1):19 [53] Tedim J, Bastos A C, Kallip S, et al. Corrosion protection of AA2024?T3 by LDH conversion films. Analysis of SVET results. Electrochim Acta, 2016, 210: 215 doi: 10.1016/j.electacta.2016.05.134 [54] Zhang Y, Liu J H, Li Y D, et al. Fabrication of inhibitor anion-intercalated layered double hydroxide host films on aluminum alloy 2024 and their anticorrosion properties. J Coat Technol Res, 2015, 12(2): 293 doi: 10.1007/s11998-014-9644-1 [55] Li J, Lin K D, Luo X H, et al. Enhanced corrosion protection property of Li?Al layered double hydroxides (LDHs) film modified by 2-guanidinosuccinic acid with excellent self-repairing and self-antibacterial properties. Appl Surf Sci, 2019, 480: 384 doi: 10.1016/j.apsusc.2019.02.164 [56] Yu Y, Lu L, Li X G. Application of micro-electrochemical technologies in atmospheric corrosion of thin electrolyte layer. Chin J Eng, 2018, 40(6): 649于陽, 盧琳, 李曉剛. 微區電化學技術在薄液膜大氣腐蝕中的應用. 工程科學學報, 2018, 40(6):649 -




 下載:
下載: