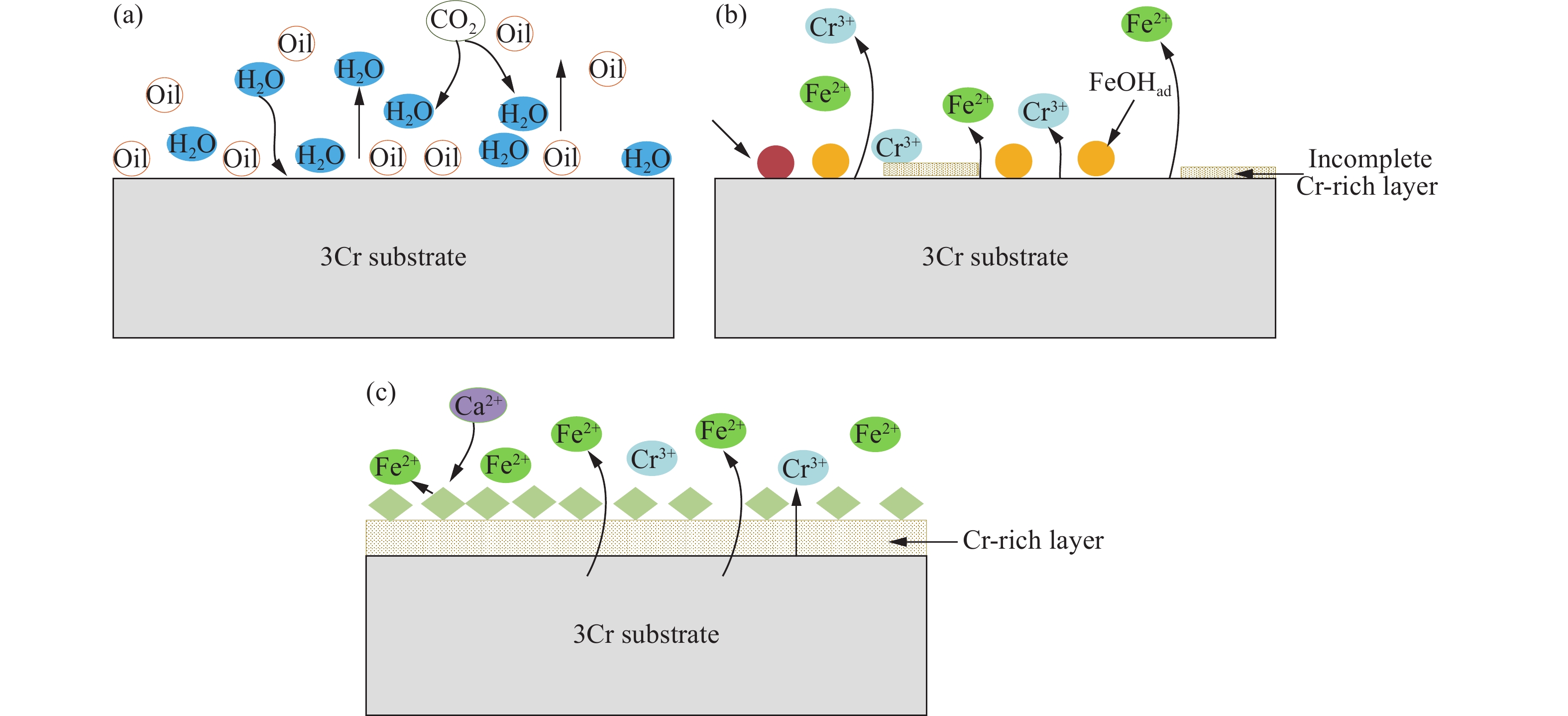
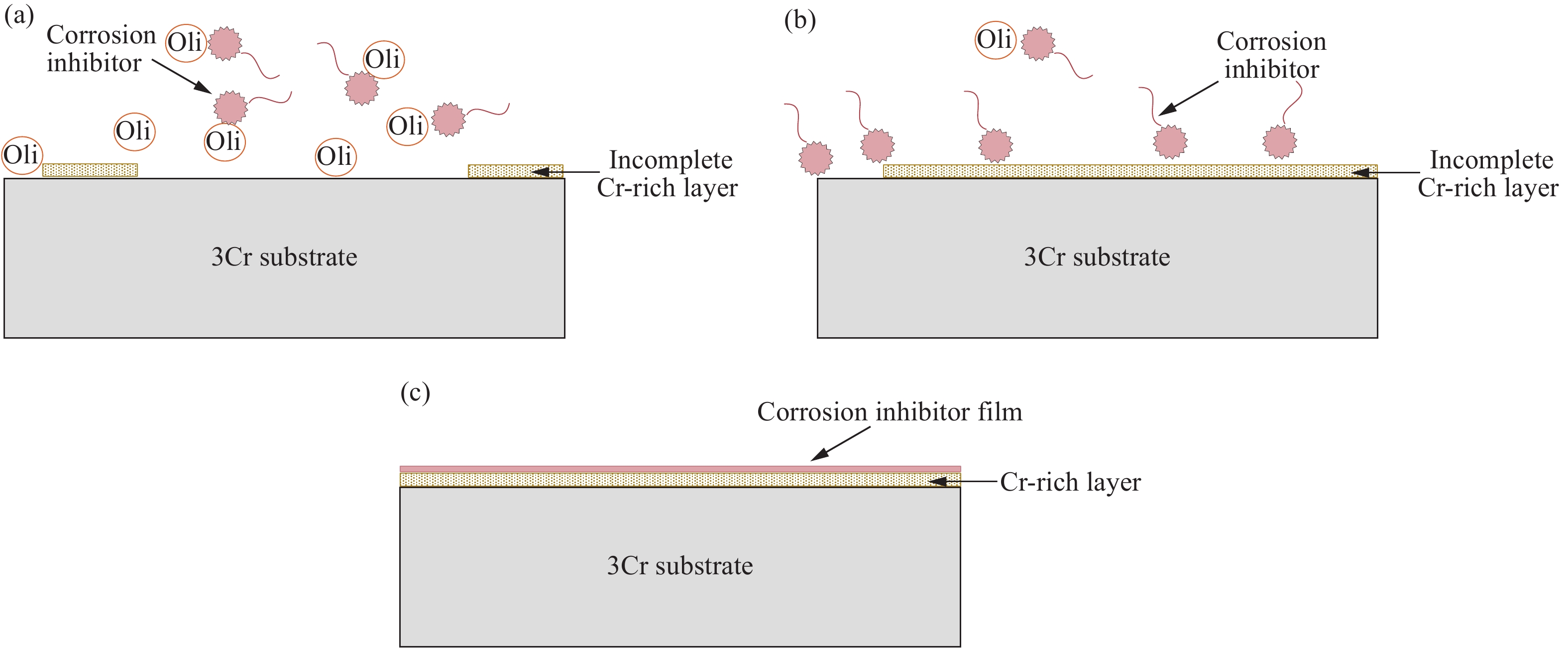
-
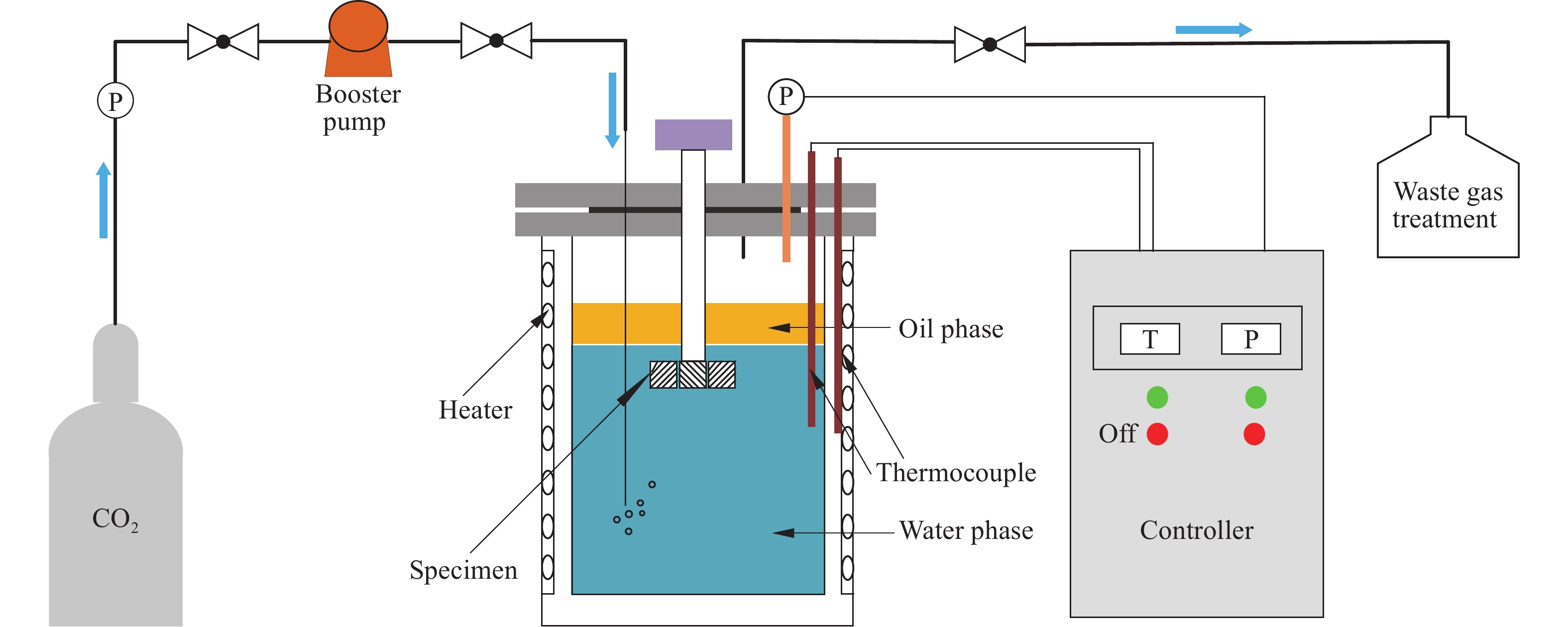
摘要: 油水兩相是海底管道和集輸管線常見的腐蝕工況之一。以3Cr鋼為代表的低Cr合金鋼是目前具有良好耐蝕性能的重要材料,但是,在油水兩相層流工況下,特別是加注了一定緩蝕劑的條件下,3Cr鋼的適用性尚不明確。通過高溫高壓反應釜模擬了油水兩相層流工況的腐蝕環境,結合掃描電子顯微鏡(SEM)、X射線衍射譜(XRD)、激光共聚焦拉曼光譜、電化學交流阻抗等測試表征方法,研究了3Cr鋼的腐蝕行為及緩蝕劑對其耐蝕性能的影響。結果表明,在油水分層工況下,3Cr鋼的腐蝕產物膜為明顯的雙層膜結構,其內層腐蝕產物膜為結構致密的富Cr層,表現出良好的抗CO2腐蝕性能,但加入100 mg·L?1十七烯基胺乙基咪唑啉季銨鹽緩蝕劑后,3Cr鋼并未得到有效的緩蝕保護。腐蝕產物分析和電化學研究表明,烷烴分子、緩蝕劑分子及富Cr層間存在競爭關系,烷烴分子干擾了緩蝕劑分子的有序排列,影響了3Cr鋼的耐蝕性。Abstract: With the growing of CO2 corrosion problem in multiphase oil and gas in-field pipelines, carbon steel can no longer meet the continuously growing demand for energy consumption. At the same time, the water content in the gathering pipelines and the complex phase distribution of the oil and water phases make the service environment of the pipeline steel increasingly demanding. Recently, the low Cr-containing steel, which shows an excellent performance-price ratio with a better CO2 corrosion resistance, is expected to replace the carbon steel used for pipelines. However, the application of 3Cr is limited under the conditions of oil-water flows, especially those with corrosion inhibitor. For example, the absolute value of the uniform corrosion rate is still relatively high in environments of high-carbon dioxide, and using corrosion inhibitor in the application of Cr-containing low-alloy steels is still necessary. Some researchers found that the corrosion inhibitor of imidazoline quaternary ammonium salt can better control the corrosion caused by carbon dioxide in the application of 3Cr steel. Since the corrosion resistance of Cr-containing low-alloy steel depends on the formation of corrosion products, it is highly susceptible to corrosion inhibitors, and research on its compatibility with corrosion inhibitors is still lacking. In this study, the corrosion resistance of 3Cr steel and the effect of corrosion inhibitor on the resistance were evaluated in an oil-water two-phase environment by using a high-temperature and high-pressure autoclave combined with SEM (scanning electron microscope), XRD (X-ray diffraction), confocal Raman spectroscopy, and electrochemical impedance spectroscopy. The results show that the corrosion scales formed on the 3Cr steel consist of two layers, and the inner layer is a Cr-rich layer in this environments, exhibiting good resistance to CO2 corrosion under the conditions of oil-water flows. However, after adding 100 mg·L?1 corrosion inhibitor of seventeen alkenyl amide ethyl imidazoline quaternary ammonium salt, 3Cr steel has not been effectively protected from corrosion. The analysis of the corrosion product and electrochemical tests revealed that competition exited between alkane molecules, corrosion inhibitor molecules and Cr-rich layers and the alkanes interfered with the ordered arrangement of the corrosion inhibitor and thus affected the corrosion resistance of 3Cr steel.
-
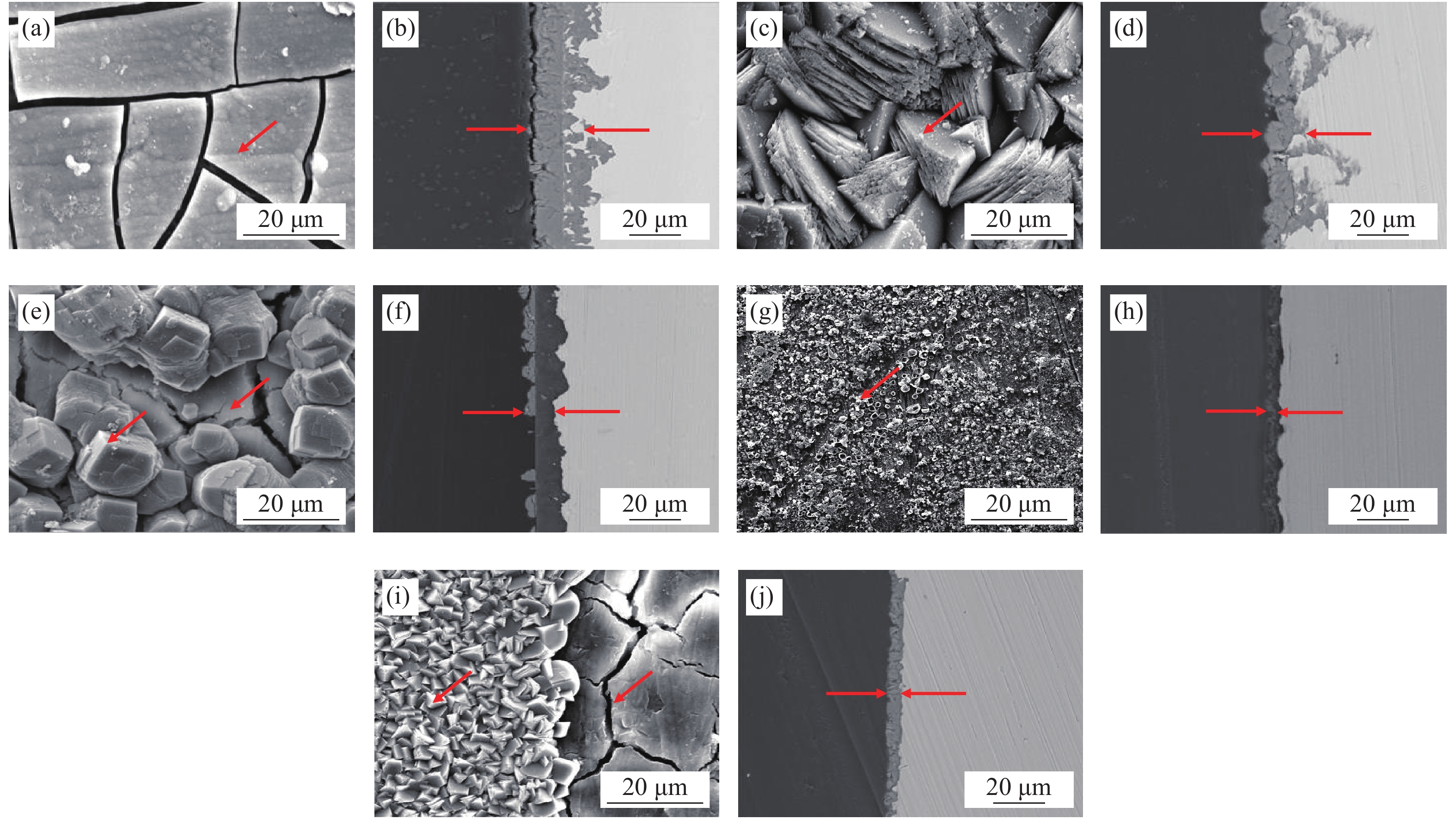
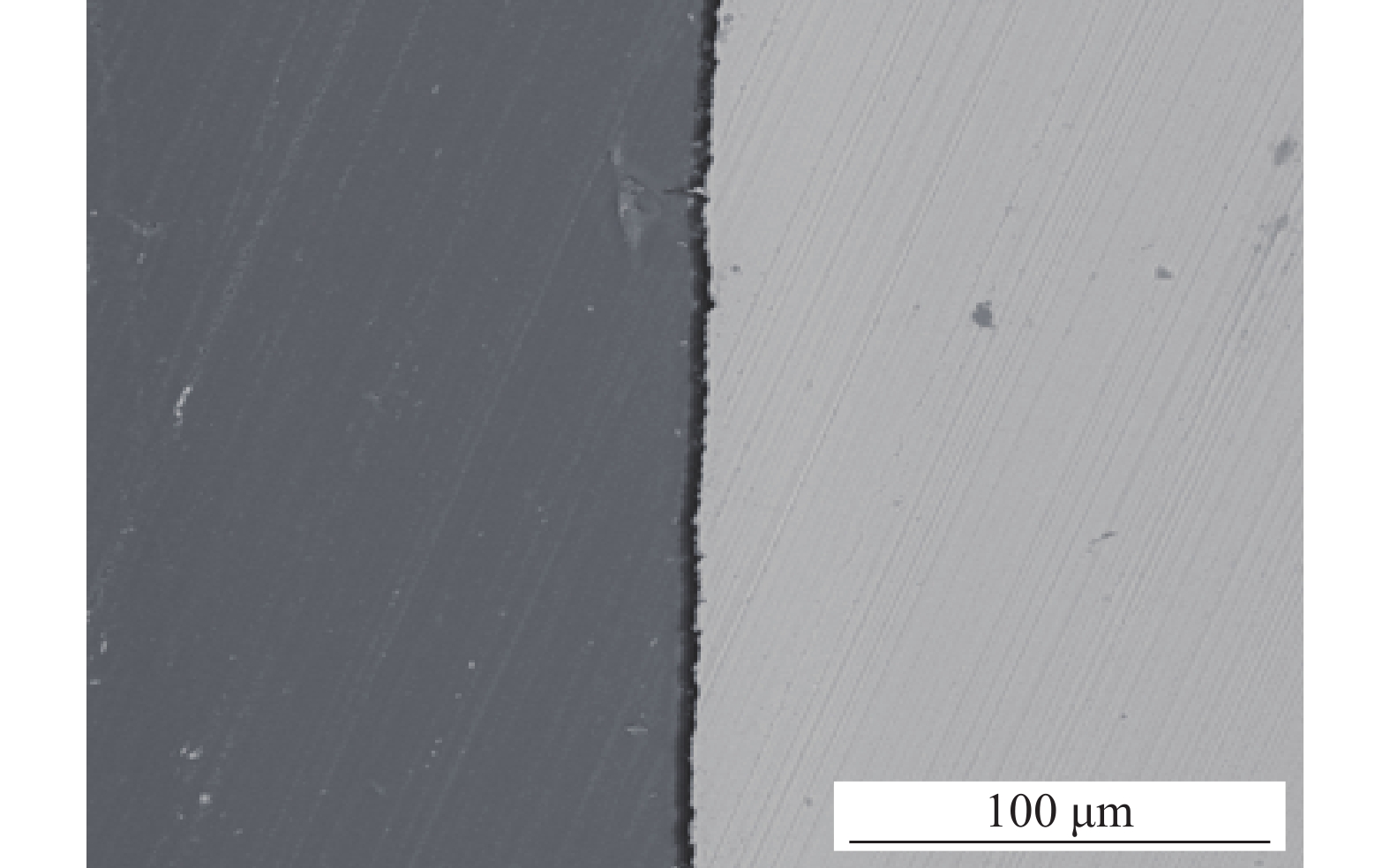
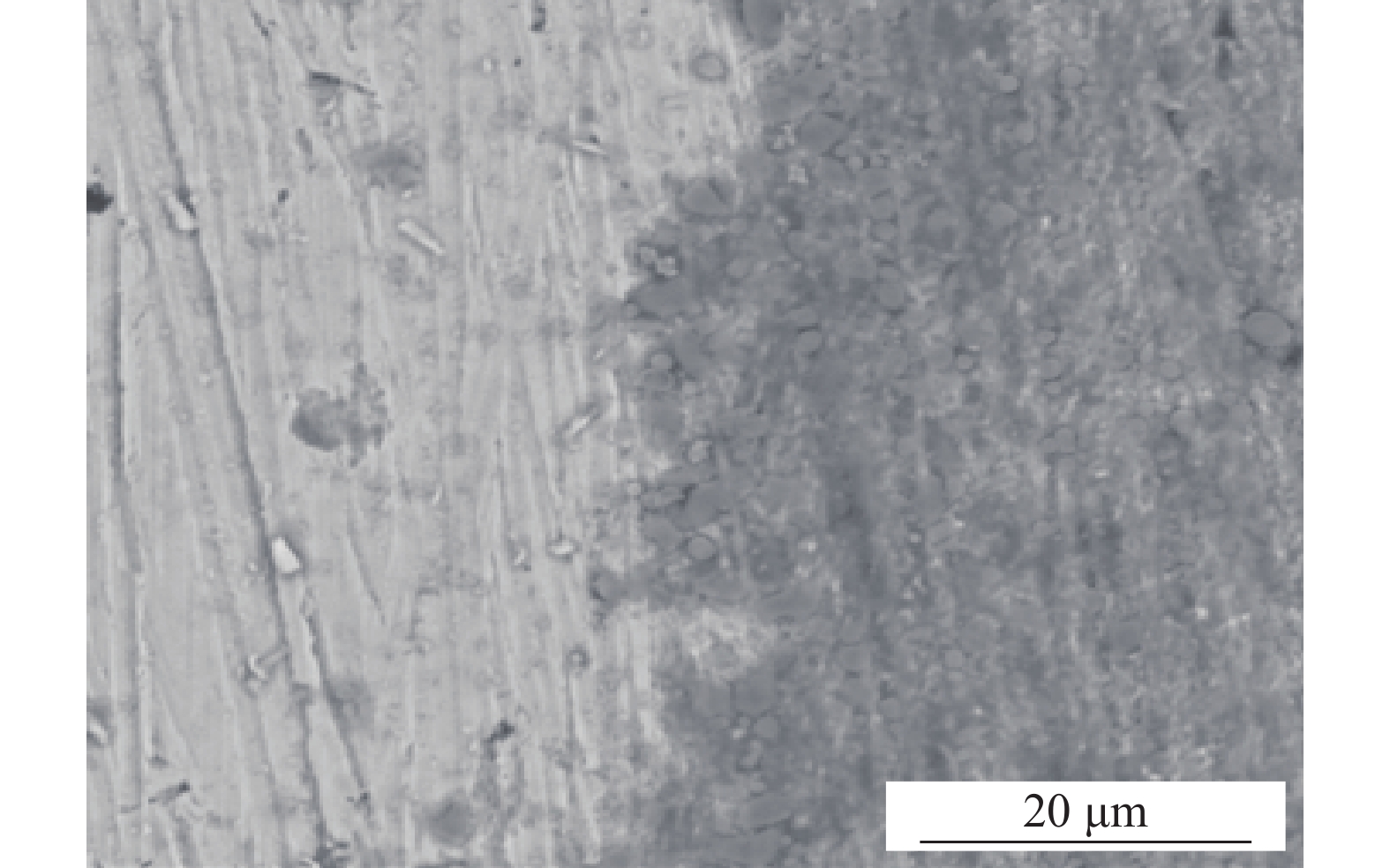
圖 5 不同環境下3Cr鋼及X65鋼腐蝕產物膜的表面形貌及截面形貌。(a)單一水相中3Cr表面;(b)單一水相中3Cr截面;(c)油水分層后的水相中X65表面;(d)油水分層后的水相中X65截面;(e)油水分層后的水相中3Cr表面;(f)油水分層后的水相中3Cr截面;(g)添加OAI緩蝕劑后X65表面;(h)添加OAI緩蝕劑后X65截面;(i)添加OAI緩蝕劑后3Cr表面;(j)添加OAI緩蝕劑后3Cr截面
Figure 5. Surface topography and sectional morphology of the corrosion product film of 3Cr and X65 steel in different environments: (a) surface morphology on 3Cr in single water phase; (b) cross section of on 3Cr in single water phase; (c) surface morphology of X65 in water phase from 10% oil mixture; (d) cross section of X65 in water phase from 10% oil mixture; (e) surface morphology of 3Cr in water phase from 10% oil mixture; (f) cross section of 3Cr in water phase from 10% oil mixture; (g) surface morphology of X65 in water phase from 10% oil mixture with OAI addition; (h) cross section of X65 in water phase from 10% oil mixture with OAI addition; (i) surface morphology of 3Cr in water phase from 10% oil mixture with OAI addition; (j) cross section of 3Cr in water phase from 10% oil mixture with OAI addition
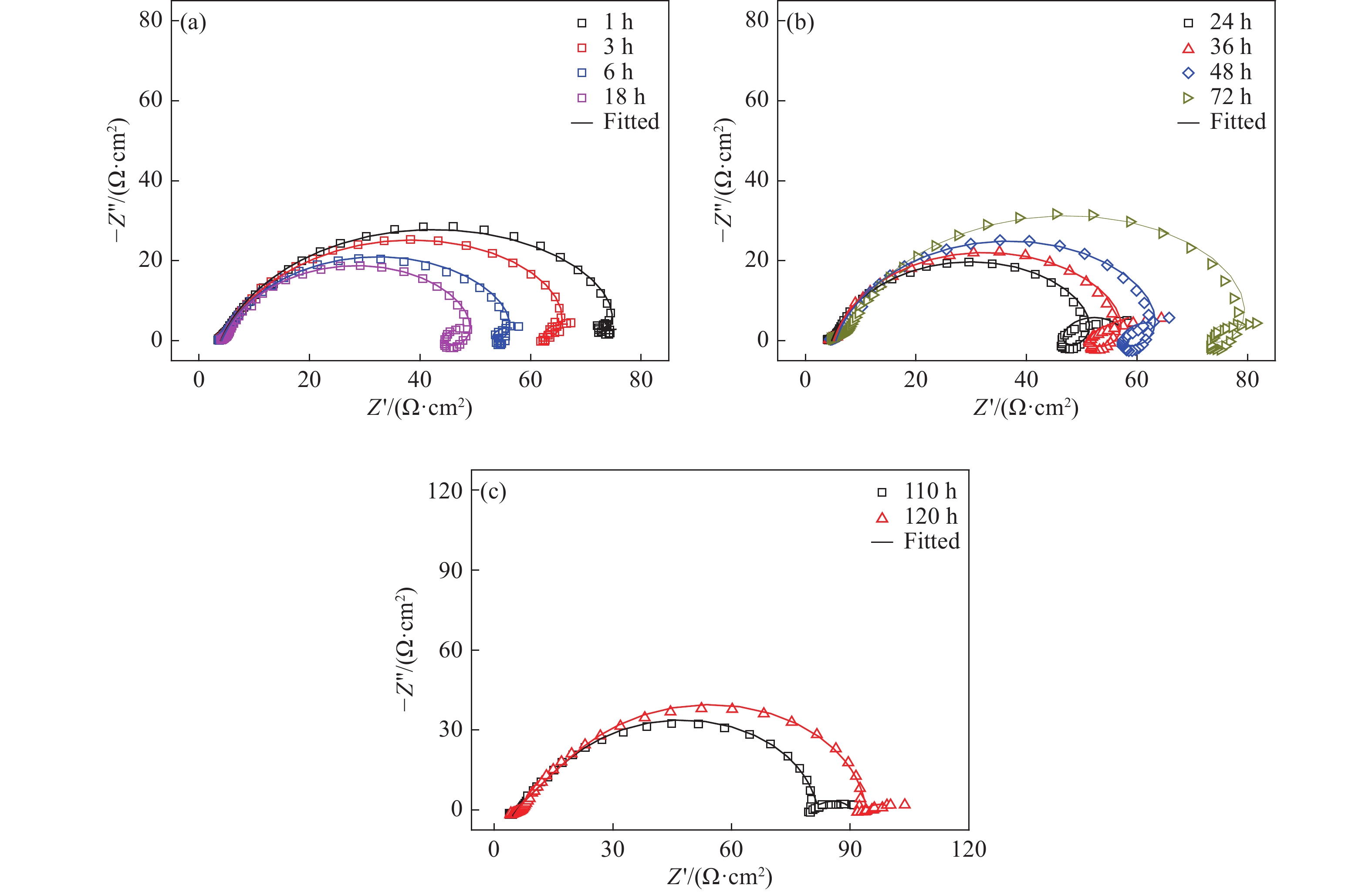
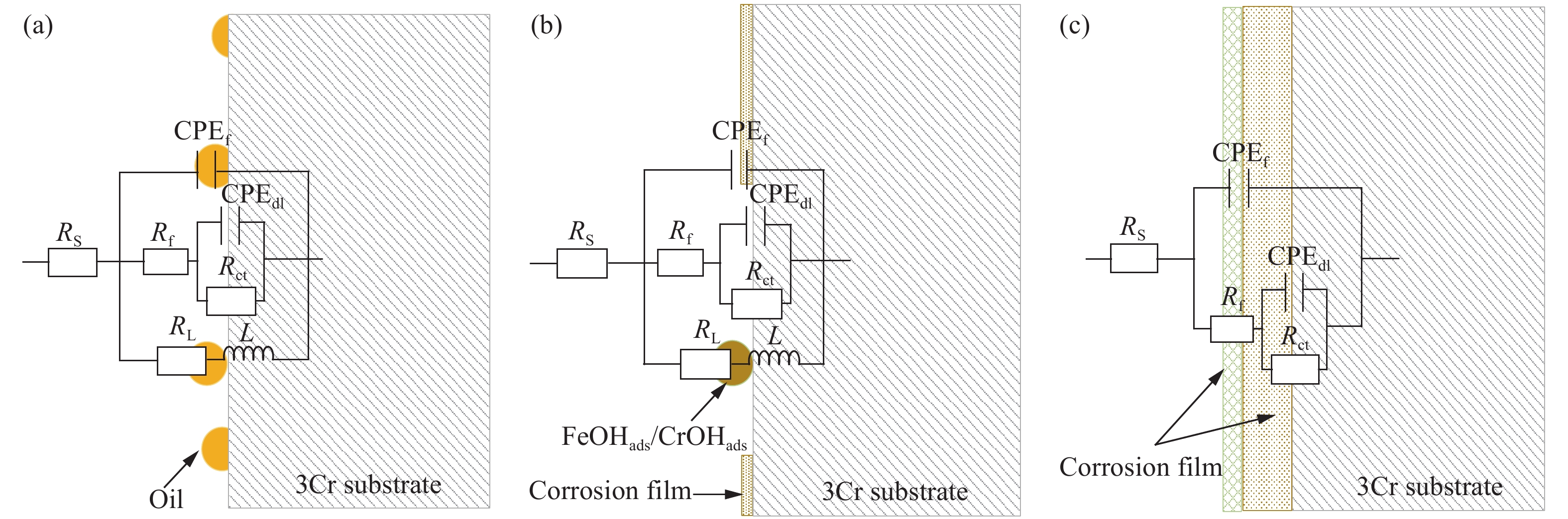
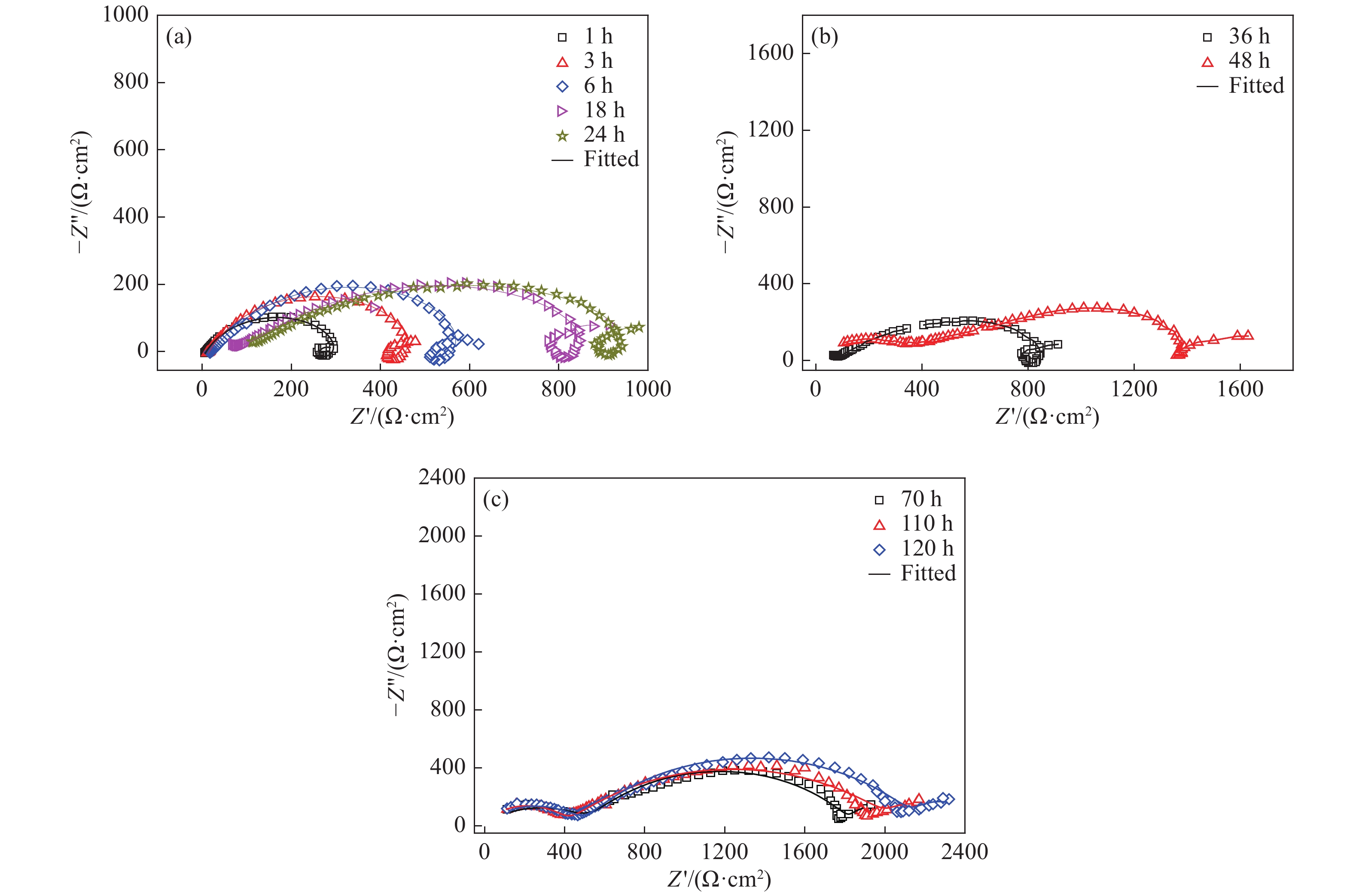
圖 11 未添加緩蝕劑時3Cr鋼在油水分層后水相區的EIS測試等效電路圖。(a)腐蝕初期(1、3、6、18 h);(b)腐蝕中期(24、36、48、72 h);(c)腐蝕后期(110、120 h)
Figure 11. Equivalent circuit used for fitting the EIS results of 3Cr steel in the aqueous phase after oil-water two phase stratification without corrosion inhibitor: (a) initial stage of corrosion(1, 3, 6, 18 h); (b) middle stage of corrosion (24, 36, 48, 72 h); (c) later stage of corrosion (110, 120 h)
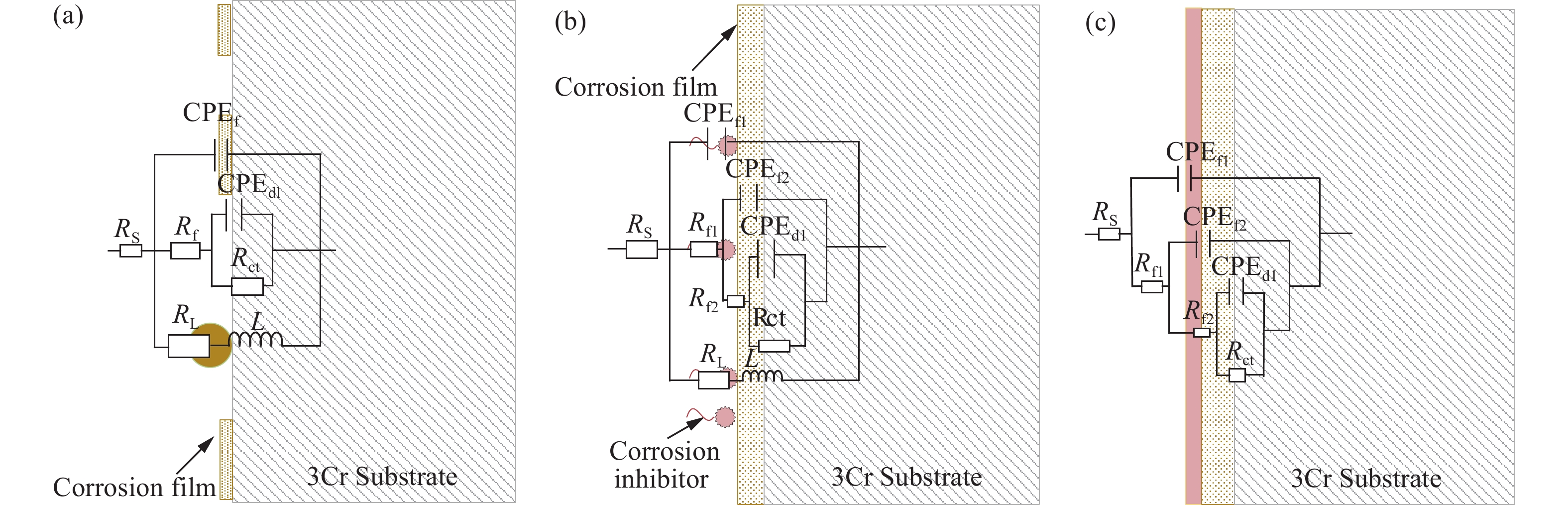
圖 13 加注100 mg?L?1緩蝕劑后3Cr鋼在油水分層后水相區的EIS測試等效電路圖。(a)腐蝕初期(1、3、6、18、24 h);(b)腐蝕中期(36、48 h);(c)腐蝕后期(72、110、120 h)
Figure 13. Equivalent circuit used for fitting the EIS results of 3Cr steel in the aqueous phase after oil-water two phase stratification with 100 mg?L?1 corrosion inhibitor: (a) initial stage of corrosion (1, 3, 6, 18, 24 h); (b) middle stage of corrosion (36, 48 h); (c) later stage of corrosion (72, 110, 120 h)
圖 15 加注100 mg?L?1緩蝕劑后3Cr鋼在油水分層后水相區浸泡120 h后的腐蝕模型示意圖. (a)腐蝕初期;(b)腐蝕中期;(c)腐蝕后期
Figure 15. Schematic diagram of corrosion reaction model of 3Cr steel in the aqueous phase after oil-water two phase stratification with 100 mg?L?1 corrosion inhibitor:(a) initial stage of corrosion; (b) middle stage of corrosion; (c) later stage of corrosion
表 1 3Cr管線鋼和X65管線鋼的化學成分(質量分數)
Table 1. Chemical composition of the 3Cr and X65 pipeline steel (mass fraction)
% Elements C Mn Si Cr Mo S P Fe 3Cr 0.07 0.55 0.20 2.96 0.15 0.03 0.003 bal X65 0.04 1.50 0.20 — 0.02 0.03 0.011 bal 表 2 油田地層水采出液的組分
Table 2. Composition of the test solution simulating the oilfield formation water
mg·L?1 Ions Na+ Mg2+ Ca2+ K+ Cl- ${\rm{SO}}_4^{2-} $ ${\rm{HCO}}_3^{-} $ Concentration 26231 1920 2747 644 35297 197 519 表 3 腐蝕產物EDS測試結果(原子數分數)
Table 3. Corrosion products’ results of EDS (atomic fraction)
% Elements Fe Cr Ca O 3Cr in Single water phase 10.06 22.89 — 65.68 X65 in water phase from 10% oil mixture 20.50 — — 59.01 Inner layer of 3Cr in water phase from 10% oil mixture 6.67 18.63 3.95 52.46 Outer layer of 3Cr in water phase from 10% oil mixture 18.82 — 3.76 51.12 X65 in water phase from 10% oil mixture with OAI 44.29 — — 14.03 Inner layer of 3Cr in water phase from 10% oil mixture with OAI 20.31 6.44 4.09 54.78 Outer layer of 3Cr in water phase from 10% oil mixture with OAI 20.03 — 6.98 58.29 表 4 未添加緩蝕劑時3Cr鋼在油水分層后水相區的EIS等效電路擬合結果
Table 4. Parameter values of the equivalent circuit of EIS of 3Cr steel in the aqueous phase after oil-water two phase stratification without corrosion inhibitor
t/h Rs/(Ω·cm2) CPEf Rf/(Ω·cm2) CPEdl Rct/(Ω·cm2) RL/(Ω·cm2) L/(H·cm?2) Y1/(10?4 Ω?1·cm?2?sn) n1 Y2/(Ω?1·cm?2?sn) n2 1 3.423 6.291 0.726 1.21×10?2 0.381 1.000 71.11 12.950 1.678 3 3.714 6.599 0.750 1.00×10?2 0.626 1.000 60.81 9.845 1.588 6 4.060 7.229 0.808 48.85 0.822 1.000 6.35 8.304 2.306 18 4.497 7.499 0.840 39.34 1.205 1.000 5.41 9.225 2.526 24 4.727 7.461 0.841 41.72 1.340 1.000 11.62 9.183 2.584 36 5.072 7.160 0.850 46.54 1.983 1.000 10.91 9.853 2.962 48 5.493 7.422 0.851 51.95 3.825 1.000 9.09 11.430 3.640 72 5.979 6.533 0.815 67.23 1.739 1.000 8.31 10.680 3.530 110 5.321 6.931 0.753 21.42 1.407 1.000 61.71 120 4.874 5.806 0.508 23.30 1.351 1.000 68.45 表 5 加注100 mg?L?1緩蝕劑后3Cr鋼在油水分層后水相區的EIS等效電路擬合結果
Table 5. Parameter values of the equivalent circuit of EIS of 3Cr steel in the aqueous phase after oil-water two phase stratification with 100 mg?L?1 corrosion inhibitor
t/h Rs/(Ω·cm2) CPEf1 Rf1/(Ω·cm2) CPEf2 Y1/(10?6Ω?1·cm?2·sn) n1 Y2/(10?4Ω?1·cm?2·sn) n2 1 6.91 1.902 0.739 3 13.85 1.307 0.756 6 20.57 1.176 0.705 18 78.85 1.184 0.457 24 87.60 1.109 0.473 36 0.10 0.331 0.716 181.3 2.269 0.372 48 0.10 0.169 0.610 386.8 2.266 0.445 72 0.01 3.413 0.542 541.3 0.955 0.653 110 0.08 0.245 0.742 411.2 1.211 0.556 120 0.06 1.103 0.627 499.8 1.089 0.639 t/h Rf2/(Ω·cm2) CPEdl Rct/(Ω·cm2) RL/(Ω·cm2) L/(H·cm?2) Y3/(Ω?1·cm?2·sn) n3 1 255.3 0.282 1.000 36.90 70.1 25.52 3 397.5 0.132 1.000 72.25 103.4 46.43 6 501.2 0.084 1.000 48.85 121.1 45.66 18 846.5 0.160 1.000 137.10 219.6 86.94 24 872.6 0.153 1.000 139.30 222.9 89.85 36 957.7 0.290 1.000 175.10 351.2 194.00 48 1079.0 0.111 1.000 205.90 371.2 235.10 72 1309.0 0.151 1.000 235.50 110 1662.0 0.146 1.000 232.70 120 1687.0 0.107 1.000 271.5 259luxu-164<th id="5nh9l"></th> <strike id="5nh9l"></strike> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <strike id="5nh9l"></strike> <progress id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"><noframes id="5nh9l"> <th id="5nh9l"></th> <strike id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <progress id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"><span id="5nh9l"></span> <strike id="5nh9l"><noframes id="5nh9l"><strike id="5nh9l"></strike> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"><noframes id="5nh9l"> <span id="5nh9l"></span> <span id="5nh9l"><video id="5nh9l"></video></span> <th id="5nh9l"><noframes id="5nh9l"><th id="5nh9l"></th> <progress id="5nh9l"><noframes id="5nh9l"> -
參考文獻
[1] Pouraria H, Seo J K, Paik J K. A numerical study on water wetting associated with the internal corrosion of oil pipelines. <italic>Ocean Eng</italic>, 2016, 122: 105 doi: 10.1016/j.oceaneng.2016.06.022 [2] Gardner T, Paolinelli L D, Nesic S. Study of water wetting in oil-water flow in a small-scale annular flume. <italic>Exp Therm Fluid Sci</italic>, 2019, 102: 506 doi: 10.1016/j.expthermflusci.2018.12.010 [3] Choi H J, Tonsuwannarat T. Unique roles of hydrocarbons in flow-induced sweet corrosion of X-52 carbon steel in wet gas condensate producing wells // The 57th NACE Annual Conference. Houston, 2002: 02259 [4] Choi H J. Effect of liquid hydrocarbons on flow-induced sweet corrosion of carbon steel // The 59th NACE Annual Conference. Houston, 2004: 04664 [5] Pigliacampo L, Gonzales J C, Turconi G L. Window of application an operational track of low carbon 3Cr steel tubular // The 61th NACE Annual Conference. Houston, 2006: 06133 [6] Hu L H, Yu M L, Chang W, et al. Effect of Cr content on resistance to CO<sub>2</sub>-induced corrosion of low alloy pipeline steels. <italic>Corros Sci Prot Technol</italic>, 2011, 23(3): 256胡麗華, 俞曼麗, 常煒, 等. Cr含量對低合金管線鋼CO<sub>2</sub>腐蝕性能的影響. 腐蝕科學與防護技術, 2011, 23(3):256 [7] Liu W, Lu S L, Zhang P, et al. Effect of silty sand with different sizes on corrosion behavior of 3Cr steel in CO<sub>2</sub> aqueous environment. <italic>Appl Surf Sci</italic>, 2016, 379: 163 doi: 10.1016/j.apsusc.2016.04.044 [8] Zhang L, Hu L H, Sun J B, et al. Microstructure and properties of CO<sub>2</sub> corrosion resistant low Cr pipeline steels. <italic>J Mater Eng</italic>, 2009(5): 6 doi: 10.3969/j.issn.1001-4381.2009.05.002張雷, 胡麗華, 孫建波, 等. 抗CO<sub>2</sub>腐蝕低Cr管線鋼組織和性能研究. 材料工程, 2009(5):6 doi: 10.3969/j.issn.1001-4381.2009.05.002 [9] Guo S Q, Xu L N, Zhang L, et al. Corrosion of alloy steels containing 2% chromium in CO<sub>2</sub> environments. <italic>Corros Sci</italic>, 2012, 63: 246 doi: 10.1016/j.corsci.2012.06.006 [10] Xie Y, Xu L N, Gao C L, et al. Corrosion behavior of novel 3%Cr pipeline steel in CO<sub>2</sub> top-of-line corrosion environment. <italic>Mater Des</italic>, 2012, 36: 54 doi: 10.1016/j.matdes.2011.11.003 [11] Xu L N, Zhu J Y, Xie Y, et al. Mechanical properties and corrosion behavior of Cr containing pipeline steel. <italic>J Univ Sci Technol Beijing</italic>, 2014, 36(2): 200許立寧, 朱金陽, 謝云, 等. 含Cr管線鋼的力學性能和耐蝕性能. 北京科技大學學報, 2014, 36(2):200 [12] Kermani B, Gonzales J C, Turconi G L, et al. In-field corrosion performance of 3% Cr steels in sweet and sour downhole production and water injection // Corrosion 2004. New Orleans, 2004: NACE-04111 [13] Chen C F, Lu M X, Sun D B, et al. Effect of chromium on the pitting resistance of oil tube steel in a carbon dioxide corrosion system. <italic>Corrosion</italic>, 2005, 61(6): 594 doi: 10.5006/1.3278195 [14] Hua Y, Mohammed S, Barker R, et al. Comparisons of corrosion behaviour for X65 and low Cr steels in high pressure CO<sub>2</sub>-saturated brine. <italic>J Mater Sci Technol</italic>, 2020, 41(15): 21 [15] Wei L, Gao K W. Understanding the general and localized corrosion mechanisms of Cr-containing steels in supercritical CO<sub>2</sub>-saturated aqueous environments. <italic>J Alloys Compd</italic>, 2019, 792(5): 328 [16] Zhu J Y, Xu L N, Lu M X, et al. Essential criterion for evaluating the corrosion resistance of 3Cr steel in CO<sub>2</sub> environments: prepassivation. <italic>Corros Sci</italic>, 2015, 93: 336 doi: 10.1016/j.corsci.2015.01.030 [17] Wang L. Microstructure and Ion Selectivity of CO2 Corrosion Product Films [Dissertation]. Beijing: China University of Petroleum, Beijing, 2007王磊. CO2腐蝕產物膜微觀結構與離子選擇性研究[學位論文]. 北京: 中國石油大學(北京), 2007 [18] Wang K, Yin Z F, Yang F, et al. Comparison of anti-corrosion properties of pipeline steels between 3Cr and 20<sup>#</sup> under simulated CO<sub>2</sub> flooding environment. <italic>Total Corros Control</italic>, 2013, 27(7): 45 doi: 10.3969/j.issn.1008-7818.2013.07.012王珂, 尹志福, 楊帆, 等. 模擬CO<sub>2</sub>驅環境下3Cr和20#集輸管線鋼防腐性能對比. 全面腐蝕控制, 2013, 27(7):45 doi: 10.3969/j.issn.1008-7818.2013.07.012 [19] Wang K, Zhang Y Q, Yin Z F, et al. Corrosion behavior of N80 and 3Cr tubing steels in CO<sub>2</sub> flooding environment. <italic>Corros Prot</italic>, 2015, 36(8): 706 doi: 10.11973/fsyfh-201508003王珂, 張永強, 尹志福, 等. N80和3Cr油管鋼在CO<sub>2</sub>驅油環境中的腐蝕行為. 腐蝕與防護, 2015, 36(8):706 doi: 10.11973/fsyfh-201508003 [20] Kee K E, Richter S, Babic M, et al. Experimental study of oil-water flow patterns in a large diameter flow loop—the effect on water wetting and corrosion. <italic>Corrosion</italic>, 2015, 72(4): 569 [21] Cui L, Gao Y, Gu C S, et al. Effect of trace element Cr on microstructures and properties of welded Joints of marine corrosion resisting steels. <italic>J Beijing Univ Technol</italic>, 2018, 44(6): 953崔麗, 高艷, 顧長石, 等. 微量元素Cr對船用耐蝕鋼焊接接頭組織和性能的影響. 北京工業大學學報, 2018, 44(6):953 [22] Wang B, Xu L N, Liu G Z, et al. Corrosion behavior and mechanism of 3Cr steel in CO<sub>2</sub> environment with various Ca<sup>2+</sup> concentration. <italic>Corros Sci</italic>, 2018, 136: 210 doi: 10.1016/j.corsci.2018.03.013 [23] Lin X Q. Corrosion Behavior of Carbon steel and Low Alloy Steel in CO2 and O2 High Temperature and High Pressure Environment of Oil and Gas Fields[Dissertation]. Beijing: University of Science and Technology Beijing, 2015林學強. 碳鋼和低合金鋼在含O2高溫高壓CO2油氣田環境中腐蝕行為研究[學位論文]. 北京: 北京科技大學, 2015 [24] Liu J X, Zhou Z L, Cao Z Q, et al. Corrosion properties of Cu-50Co bulk alloys in NaCl solution. <italic>Chin J Rare Met</italic>, 2020, 44(2): 127劉嘉欣, 周子力, 曹中秋, 等. Cu-50Co塊體合金在NaCl溶液中的腐蝕性能研究. 稀有金屬, 2020, 44(2):127 [25] Zhu J Y, Xu L N, Lu M X. Electrochemical impedance spectroscopy study of the corrosion of 3Cr pipeline steel in simulated CO<sub>2</sub>-saturated oilfield formation waters. <italic>Corrosion</italic>, 2015, 71(7): 854 doi: 10.5006/1494 [26] Li C, Richter S, Nesic S. How do inhibitors mitigate corrosion in oil-water two-phase flow beyond lowering the corrosion rate. <italic>Corrosion</italic>, 2014, 70(9): 958 doi: 10.5006/1057 [27] Zhu J Y, Xu L N, Lu M X, et al. Interaction effect between Cr(OH)<sub>3</sub> passive layer formation and inhibitor adsorption on 3Cr steel surface. <italic>RSC Adv</italic>, 2015, 5(24): 18518 doi: 10.1039/C4RA15519J -




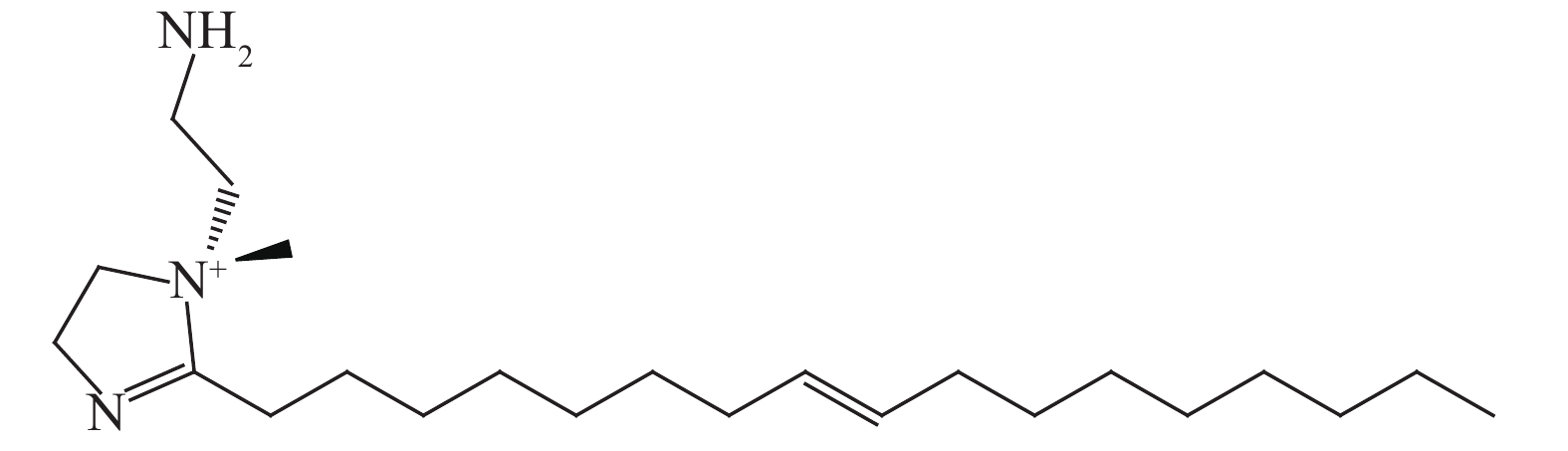
 下載:
下載: